by Juan C. Linares Casas - Alberto J. Muniagurria - Eduardo Baravalle
The physician, when examining the precordium, should stand on the right side of the patient. From the right it is possible to carry out the inspection and palpation maneuvers successively, comfortably, and with a wide view of the jugular venous pulse. Furthermore, the observer's right hand takes the same shape as that of the patient's heart; the distal ends of the fingers at the apex and the heel of the hand at the base of the heart.
The patient must be in supine position, 45 °, with the head slightly rotated to the left and breathing calmly; and during the examination you will be induced to adopt the left lateral decubitus position, squatting, standing, coughing or breathing in some special way according to the sign to be highlighted.
As a general rule, positive or outward beats are best assessed by palpation, while negative beats are best assessed by inspection.
 VENOUS PULSE
VENOUS PULSE
In the jugular vein examination, it is possible to estimate the venous pressure in the right atrium and demonstrate or rule out those causes that increase said pressure, such as right heart failure or cardiac tamponade due to a pericardial effusion. It can also be useful to guide the diagnosis of arrhythmias such as atrioventricular block and atrial fibrillation. It also makes it possible to assess the degree of severity of pulmonary stenosis or pulmonary hypertension and suggest the diagnosis of tricuspid valve disease.
If there is an obstruction of the superior vena cava, there will be no venous pulse; Hepatomegaly will not be palpable and there will be no abdominojugular reflux.
To investigate this reflux, the examiner exerts sustained pressure on the upper abdomen with both hands for thirty seconds. If the end-diastolic pressure of the right ventricle is elevated, the ventricle will not be in a position to receive a new blood supply and, therefore, sustained jugular engorgement will occur (positive reflux).
The patient should breathe calmly during this maneuver. This reflux will be positive in right heart failure and constrictive pericarditis
By observing the venous pulse waves, the following information can be obtained:
- Waves to large and prominent, which can be regular or irregular.
- 2. Disappearance of the wave a.
- Prominent cv wave or tricuspid regurgitation.
- M wave o constrictive pericarditis.
Waves to large and prominent . Waves a large, prominent and regular. Any cause that alters the normal filling of the right ventricle will produce a presystole with greater "contraction force". The right atrium tries to overcome the obstacle to the blood restriction and in fighting it will produce a large, prominent and regular pressure wave when the heart rate remains regular.
The mentioned obstacle can be present at different levels and, if the path of the cardiopulmonary central blood circulation is traversed from the left ventricle backwards, it is enough to remember the possible pathologies to find the causes of slowing or obstruction to normal hemodynamic flow.
 Cardiomyopathy or primary myocardial disease, mitral disease, left atrial myxoma, pulmonary thromboembolism and pulmonary hypertension produce large, prominent and regular a waves when pulmonary hypertension is marked, whereas right atrial myxoma and pulmonary valve stenosis produce this phenomenon in manifest form.
Cardiomyopathy or primary myocardial disease, mitral disease, left atrial myxoma, pulmonary thromboembolism and pulmonary hypertension produce large, prominent and regular a waves when pulmonary hypertension is marked, whereas right atrial myxoma and pulmonary valve stenosis produce this phenomenon in manifest form.
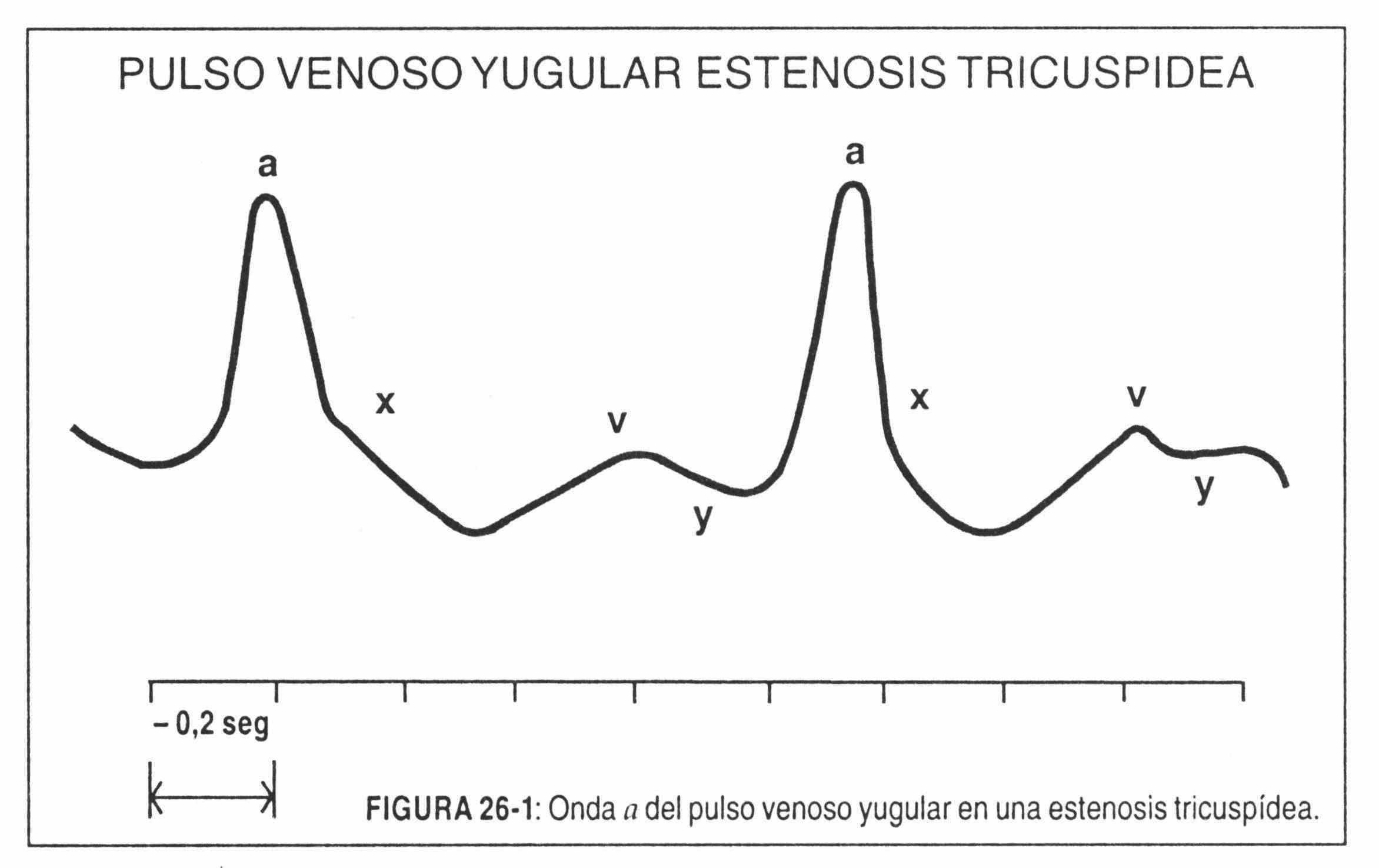
The a wave is large because the compliance of the right ventricle is decreased and the atrium must contract with greater force in order to empty its blood into the ventricle. This wave may be prominent if the pathology corresponds to tricuspid atresia or stenosis (Figure 26-1). In these circumstances the negative y wave is gradual and prolonged.
In the aforementioned cases, the a wave becomes the most important of the jugular venous pulse.
Waves a large, prominent and irregular . When there is an alteration of the heart rhythm, the wave a will be variable in its appearance, prominent in some beats and normal in others.
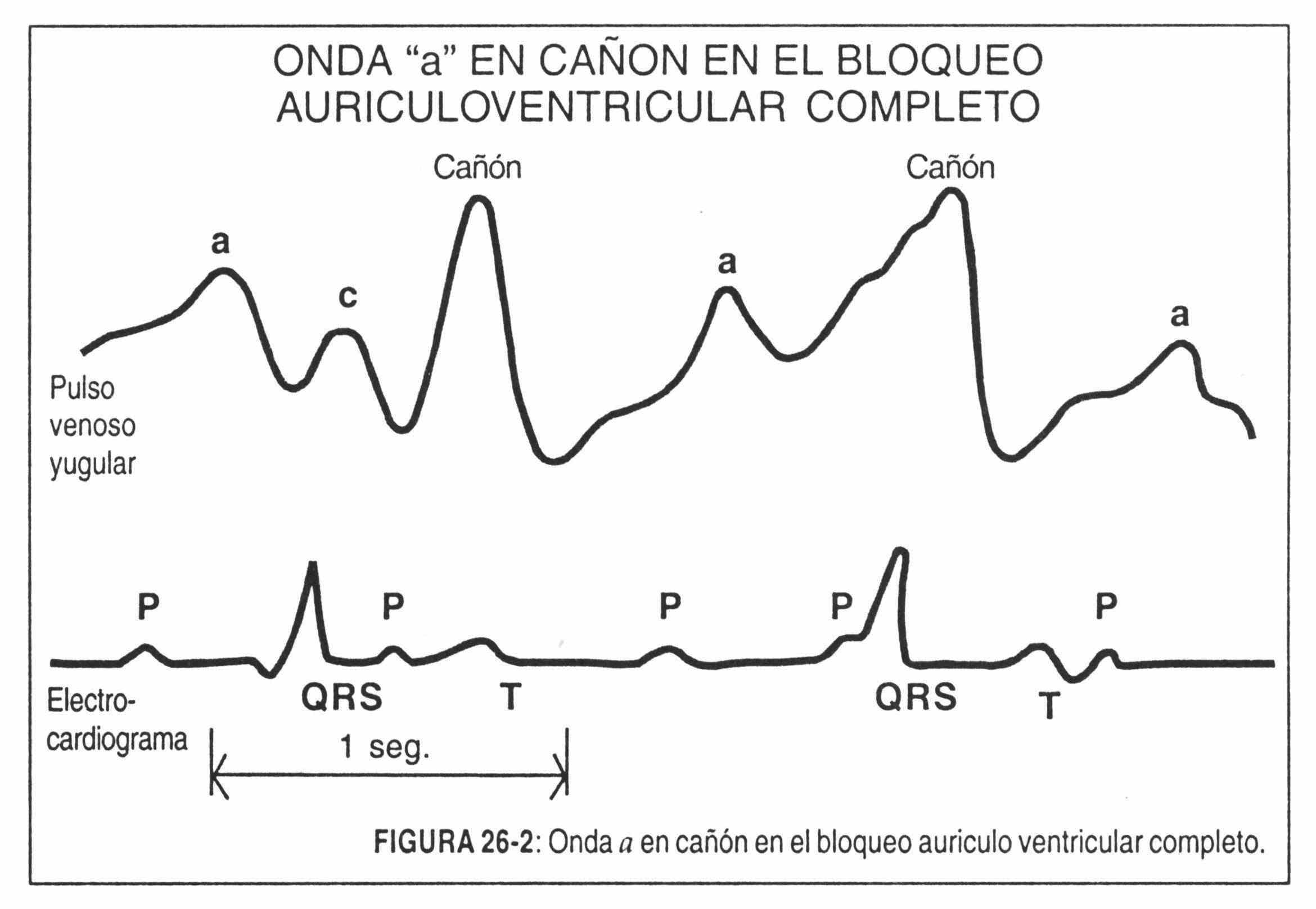
In the case of AV junctional rhythms or prolonged AV conduction time, the atrium may contract at times when the tricuspid valve is closed. Upon finding its closed path, the blood flows back and a prominent a-wave or giant a-wave is produced (Figure 26-2). This phenomenon is seen more frequently in complete AV blocks (Figure 26-2). In this situation, the a wave is of normal size except when the atrial contraction finds the tricuspid valve closed. In the case of extrasystoles or premature beats, when the contraction of the atrium finds the tricuspid valve closed, the same phenomenon occurs.
No wave a . The a wave does not occur in atrial fibrillation, due to the lack of periodic contraction of atrial systole.
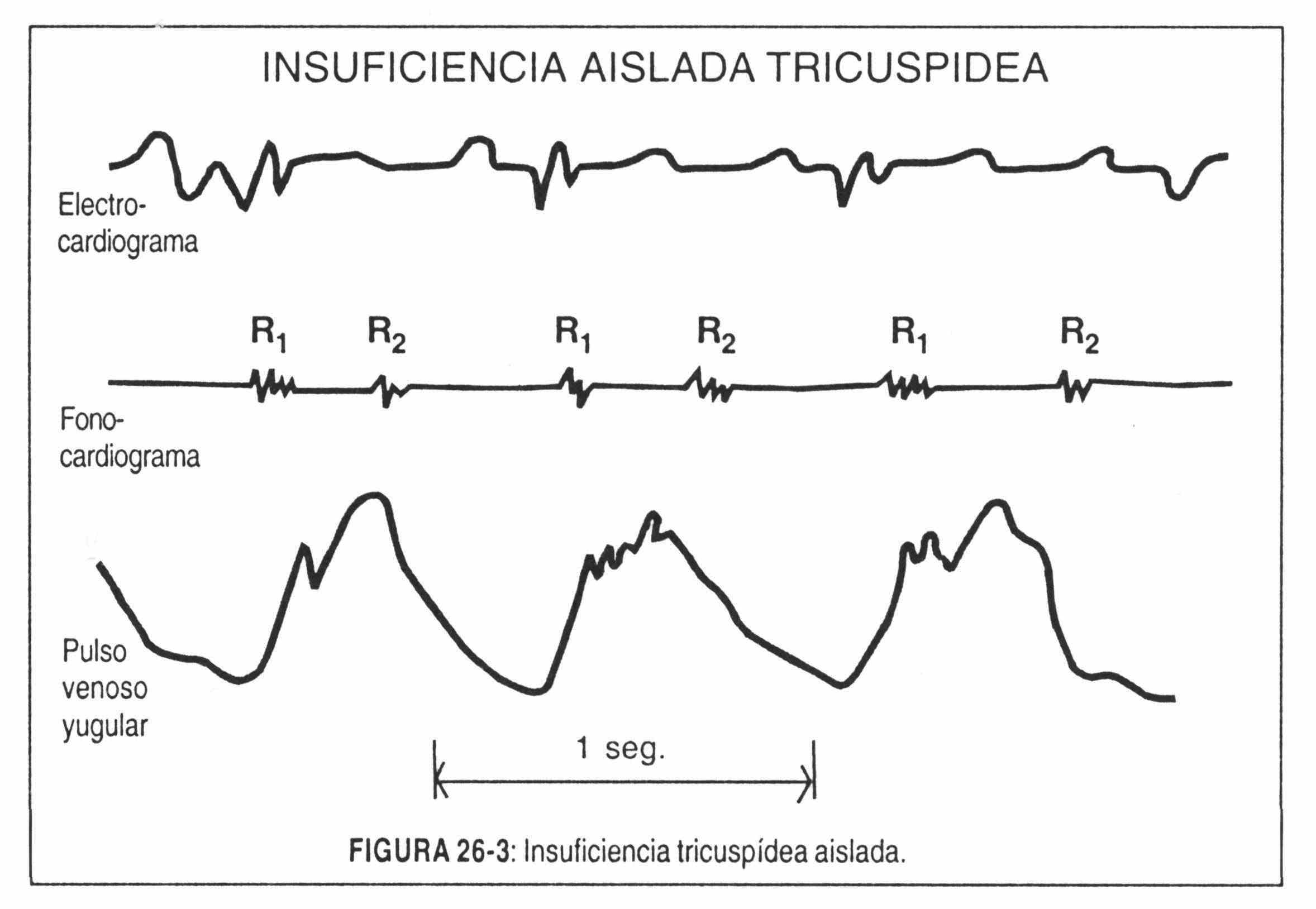
Prominent cv wave . There are patients who present a tricuspid valve injury or alteration in its shape. The most common cause is global heart failure, in which the dilation of the right chambers alters the normal shape of the valve by traction of its pillars with blood regurgitation during systole. The alterations can also be produced by rheumatic disease or tricuspid bacterial endocarditis. The latter usually occurs in drug-addicted patients whose right valves become infected by repeated intravenous injections.
The wave that dominates the tracing is the so-called "cv regurgitation wave". It can be very large and is present even in patients with atrial fibrillation. It is the manifestation of systolic expulsion of the right ventricle (Figure 26-3).
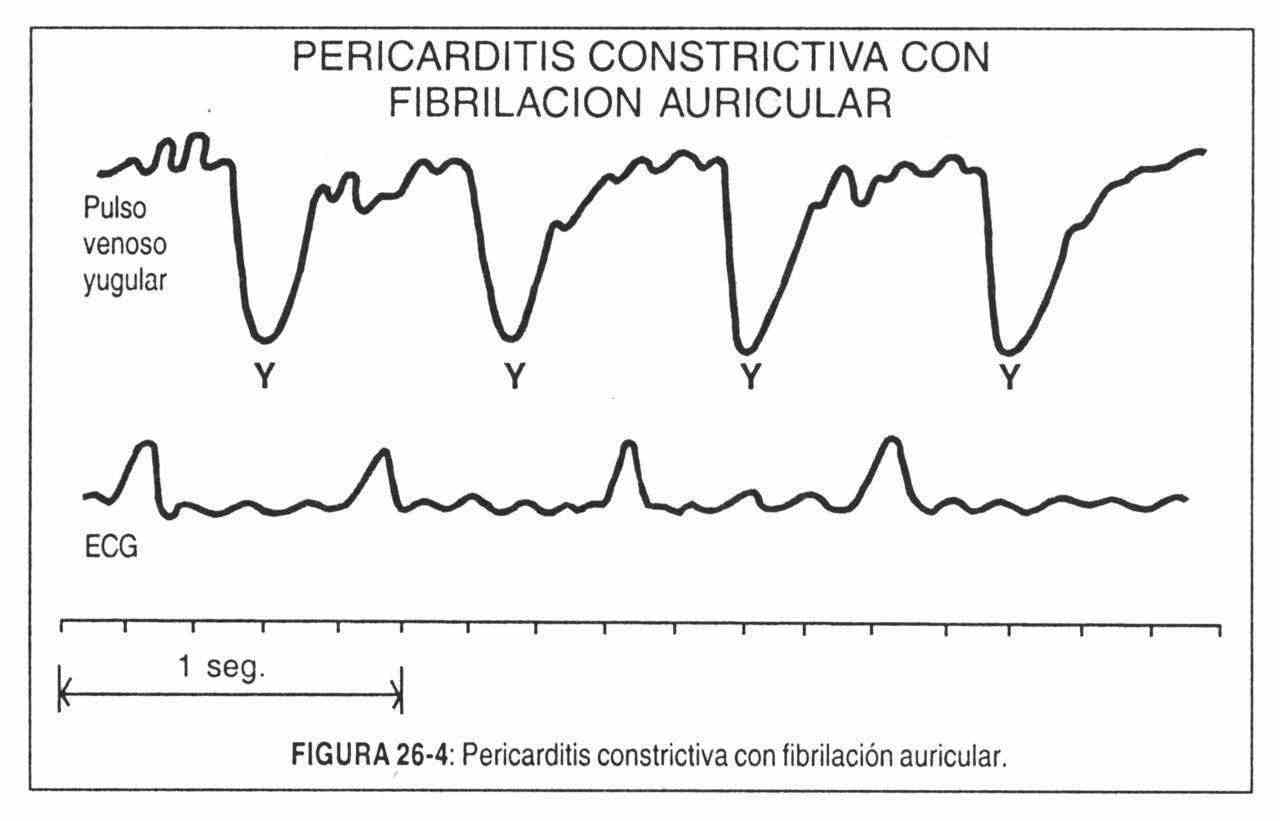
M wave . In constrictive pericarditis, or in isolated cases in cardiac tamponade, venous engorgement is maintained regardless of the position of the patient. Despite diuretic treatment and improvement in the clinical picture, engorgement persists.
In addition there are two characteristic signs. Kussmaul's sign characterized by venous distention of the neck on inspiration and increased pulsation in the neck. This phenomenon can also be seen in global heart failure or tricuspid stenosis. The descent of xy is prominent. It produces a characteristic M-shape on the jugulogram (Figure 26-4).
 INSPECTION AND PALPATION OF THE PRECORDIO
INSPECTION AND PALPATION OF THE PRECORDIO
On examination of the precordium, systolic, diastolic, positive or negative movements, thrills (sustained high-frequency vibrations), and heart sounds (short, high-frequency vibrations) may appear.
Sternoclavicular joint area
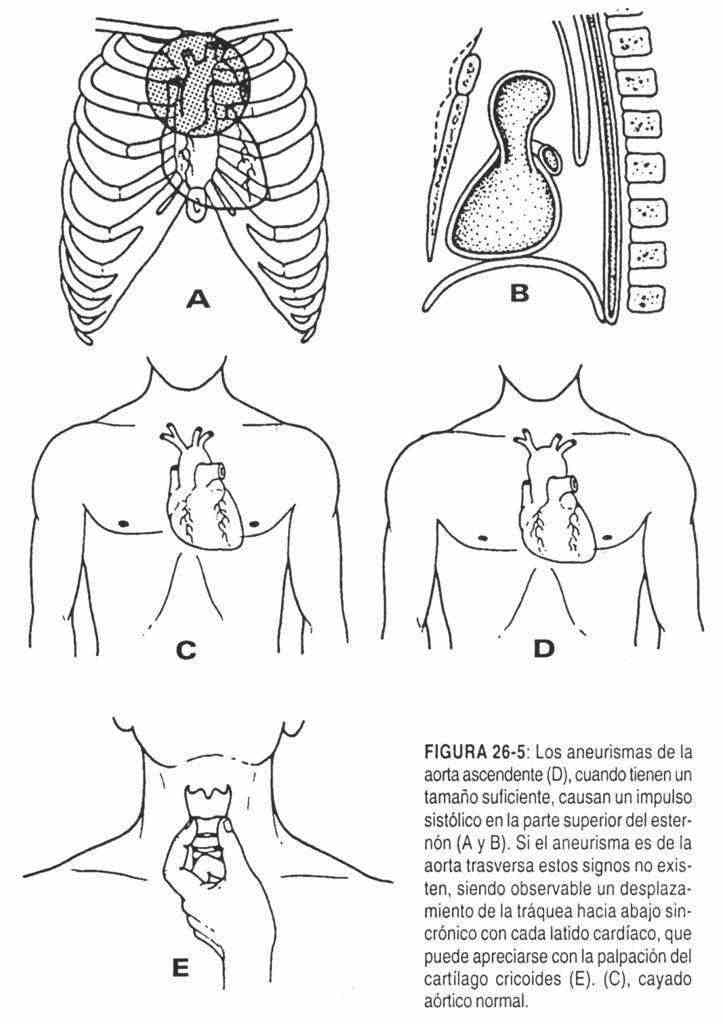
Pulsation is not normally detected in this area. The appearance of abnormal movements and abnormal pulsations is related to diseases of the aorta, aneurysmal dilations due to arteriosclerosis and dissecting processes, or of syphilitic origin. On some occasions, in patients with dilated right aortic arches, movements can be seen at the level of the sternoclavicular joints, and at the level of the sternal manubrium in tetralogy of Fallot.
Aortic area
In the aortic area, the palpation of abnormal pulsations is produced by dilations of the ascending aorta, aneurysms, or aortic regurgitation (Figure 26-5). In other words, palpation of a beat in the right first or second intercostal space should suggest an increase in the size of the ascending aorta or an ascending aorta that is out of its usual place. It is possible to feel the vibrations produced by the aortic valves when closing (aortic component of the second sound) when these vibrations are increased, as in high blood pressure and mild aortic stenosis. To improve the possibilities of palpation, it is convenient to ask the patient to perform an expiratory apnea, with which, as the rib cage approaches the heart, this phenomenon is magnified.
Lung area
The palpation of abnormal pulsations in the pulmonary area is due to an increase in pressure in the pulmonary artery, or also to an increase in flow at the level of this artery, or, more frequently, to a combination of these two phenomena.
 Pressure in the pulmonary circuit may be increased in mitral stenosis or in primary pulmonary hypertension, which produces a slow and sustained heartbeat; while the pictures with increased flow such as atrial septal defect condition a vigorous and less sustained beat.
Pressure in the pulmonary circuit may be increased in mitral stenosis or in primary pulmonary hypertension, which produces a slow and sustained heartbeat; while the pictures with increased flow such as atrial septal defect condition a vigorous and less sustained beat.
Postteststenosis pulmonary dilations of the pulmonary valve can be palpated in this area briefly and weakly. Likewise, it is possible to palpate the vibrations of the pulmonary component of the second sound when there is an increase in pulmonary pressure.
 Right ventricular area
Right ventricular area
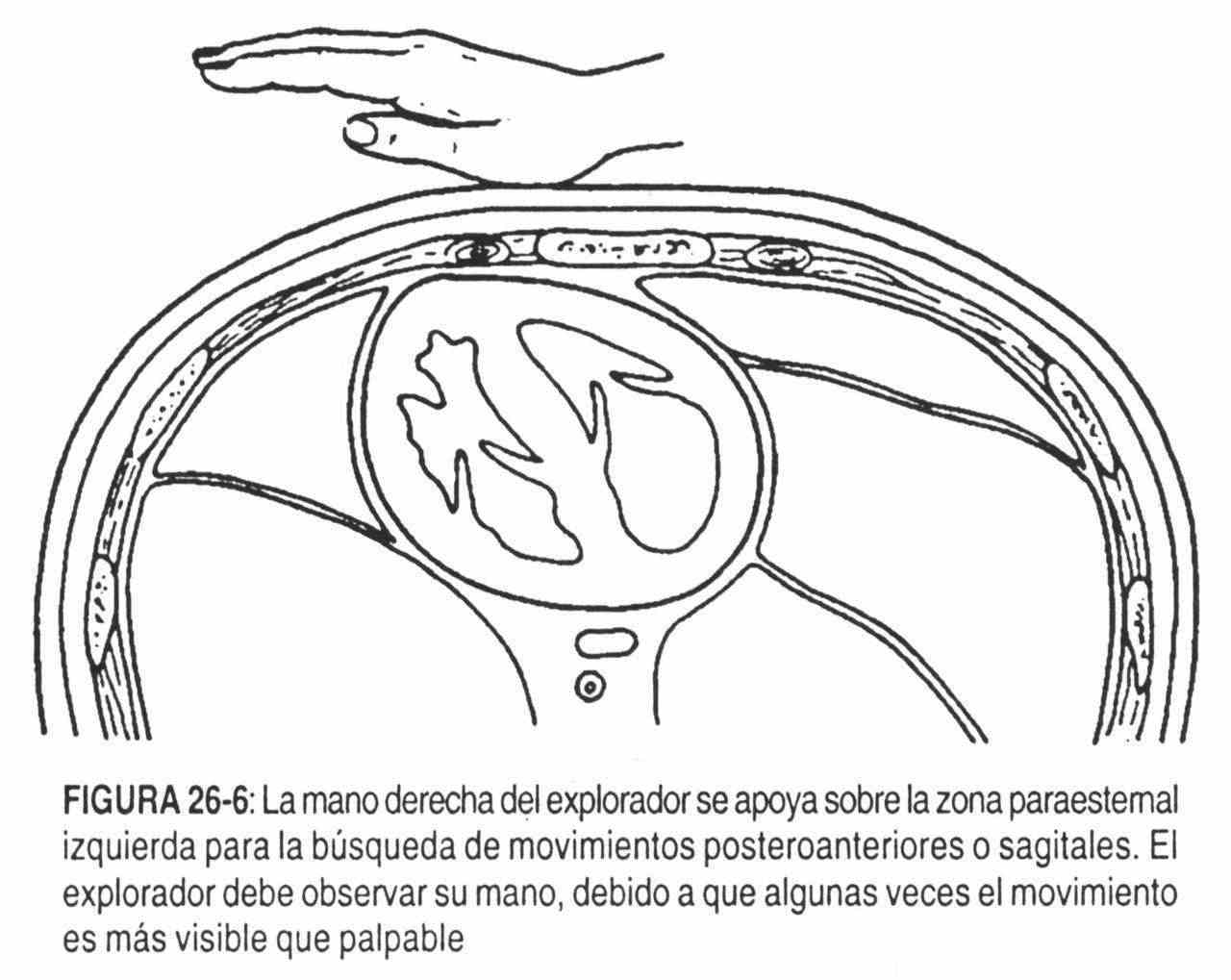
With the patient supine, the head of the bed elevated 45 °, the examiner stands on the patient's right side. Thus positioned, the examiner places the heel of his right hand in the left parasternal area of the precordium in search of a beat, called posteroanterior or sagittal. In the child with a thin chest or in the adult with sternal abnormalities, a posteroanterior systolic impulse at this level may be palpated as a normal finding (Figure 26-6).
If greater sensitivity is desired than that provided by the heel of the hand, the fingertips of the right hand are placed one in each space and intercostal, from the third to the sixth, called the right ventricular beat or sagittal beat, saving the cases mentioned. of childhood and thoracic anomalies, to which can be added the pictures in which the heartbeat is increased, as in anxiety, anemia, hyperthyroidism, fever or pregnancy, is pathological in nature. It can be produced by intrinsic or extrinsic causes of the right ventricle.
Intrinsic causes . The common intrinsic causes of the posteroanterior (sagittal) beat are right ventricular hypertrophy. In this case, the increase in size and force of contraction of the right ventricle give rise to the palpable impulse (Figure 26-7, A and B).
Semiologically, it is possible to distinguish between right ventricular hypertrophy and dilatation, that is, between right ventricular hypertension and volume overload in the right ventricle.
The first is accompanied by sustained pulsations, increased in amplitude and duration and of slower elevation, and its clearest examples are pulmonary stenosis, primary or secondary pulmonary hypertension, repeated pulmonary thromboembolism, pulmonary hypertension secondary to left heart failure and mitral stenosis.
In the second there is an increase in amplitude and speed with a normal duration of the wave or beat. Its most frequent causes are interatrial communications. This type of heartbeat can also occur in anemia, anxiety, and hyperthyroidism.
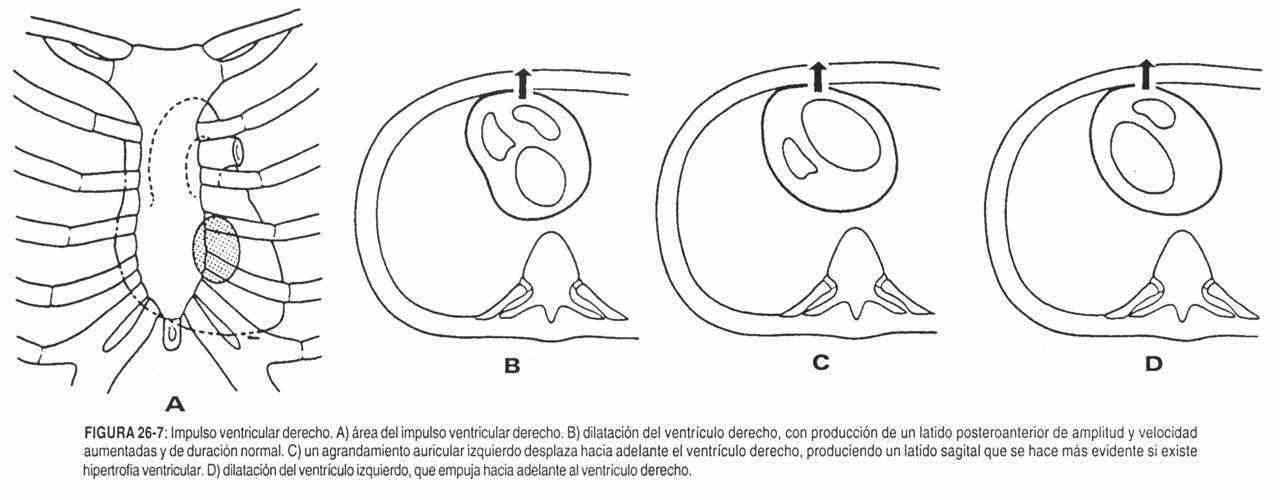 Extrinsic causes . Anatomically, the left atrium is located behind the right ventricle, between it and the thoracic spine. An enlarged left atrium pushes the right ventricle forward. Mitral disease and mitral stenosis are associated with a large left atrium. This magnifies the right ventricular systolic impulse and makes the posteroanterior beat palpable (Figure 26-7, C).
Extrinsic causes . Anatomically, the left atrium is located behind the right ventricle, between it and the thoracic spine. An enlarged left atrium pushes the right ventricle forward. Mitral disease and mitral stenosis are associated with a large left atrium. This magnifies the right ventricular systolic impulse and makes the posteroanterior beat palpable (Figure 26-7, C).
In the special case of mitral stenosis in which pulmonary hypertension and right ventricular hypertrophy are added, the impulse is doubled in amplitude. Left ventricular dilation can move the right ventricle forward (Figure 26-7. D).
The persistence in adulthood of a brief, smooth heartbeat in the fourth and fifth intercostal spaces should suggest the possibility of a tetralogy of Fallot. If a soft, brief beat persists in the third, fourth, and fifth left intercostal spaces, that is, in a larger area, it suggests a moderately increased systolic pressure, which includes the right ventricular infundibulum.
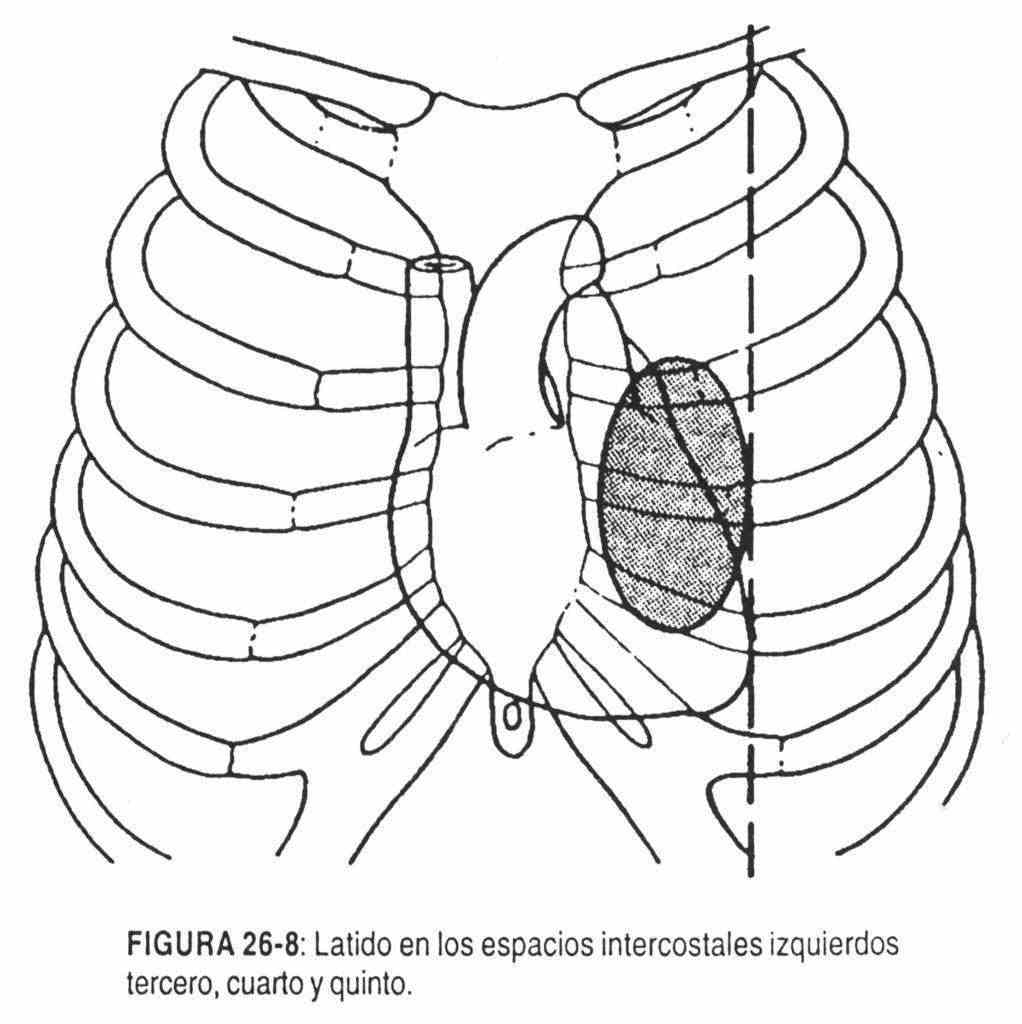 This can be seen in moderate-grade pulmonary stenosis (Figure 26-8).
This can be seen in moderate-grade pulmonary stenosis (Figure 26-8).
 Systolic retraction in the right ventricular area . The presence of a negative systolic beat, easier to examine by inspection than palpation, occurs in constrictive pericarditis. In them, the chest wall retracts with each ventricular contraction, producing a negative beat.
Systolic retraction in the right ventricular area . The presence of a negative systolic beat, easier to examine by inspection than palpation, occurs in constrictive pericarditis. In them, the chest wall retracts with each ventricular contraction, producing a negative beat.
In cardiac enlargements with global hypertrophy, a systolic propulsion is observed at the apex and parasternally, corresponding, respectively, to the contraction of the left and right ventricle.
Left Ventricular Impulse Area (IVI)
To describe the variations of the point of maximum intensity, or left ventricular impulse, the location, size and characteristics (amplitude, duration, force and speed) must be considered. In the examination of the left ventricular impulse, as in all aspects of the cardiovascular examination, it should be remembered that the physician must concentrate on a period and even a phase of the cardiac cycle, while making a correlation with other beats, such as the carotid pulse . You must also define whether the movements are diastolic or systolic in which part of diastole or systole they are.
The location of the left ventricular impulse can be modified by causes extrinsic to the heart, such as elevation of the diaphragm, pregnancy, ascites, abnormal thoracic configuration, or changes in the volume of the left lung. Sometimes the left ventricular impulse can be found in the right hemithorax due to dextrocardia.
The location will also help to evaluate the presence of left ventricular hypertrophy or dilation.
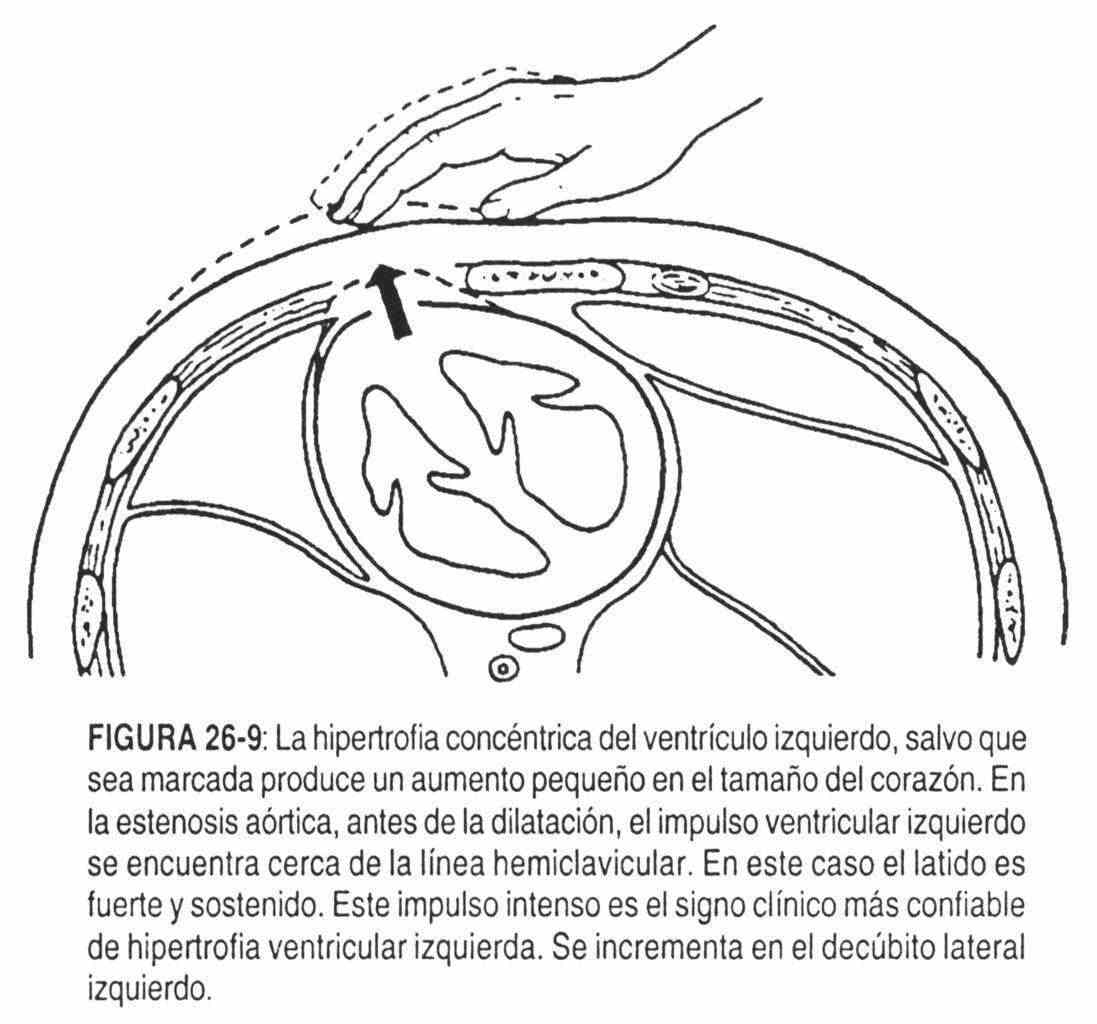
In a pure aortic stenosis with concentric hypertrophy of the left ventricle; the left ventricular impulse will be small, no greater than normal (2 cm), zero strong and with a long duration.
The location will be normal or with a very slight shift to the left (Figure 26-9).
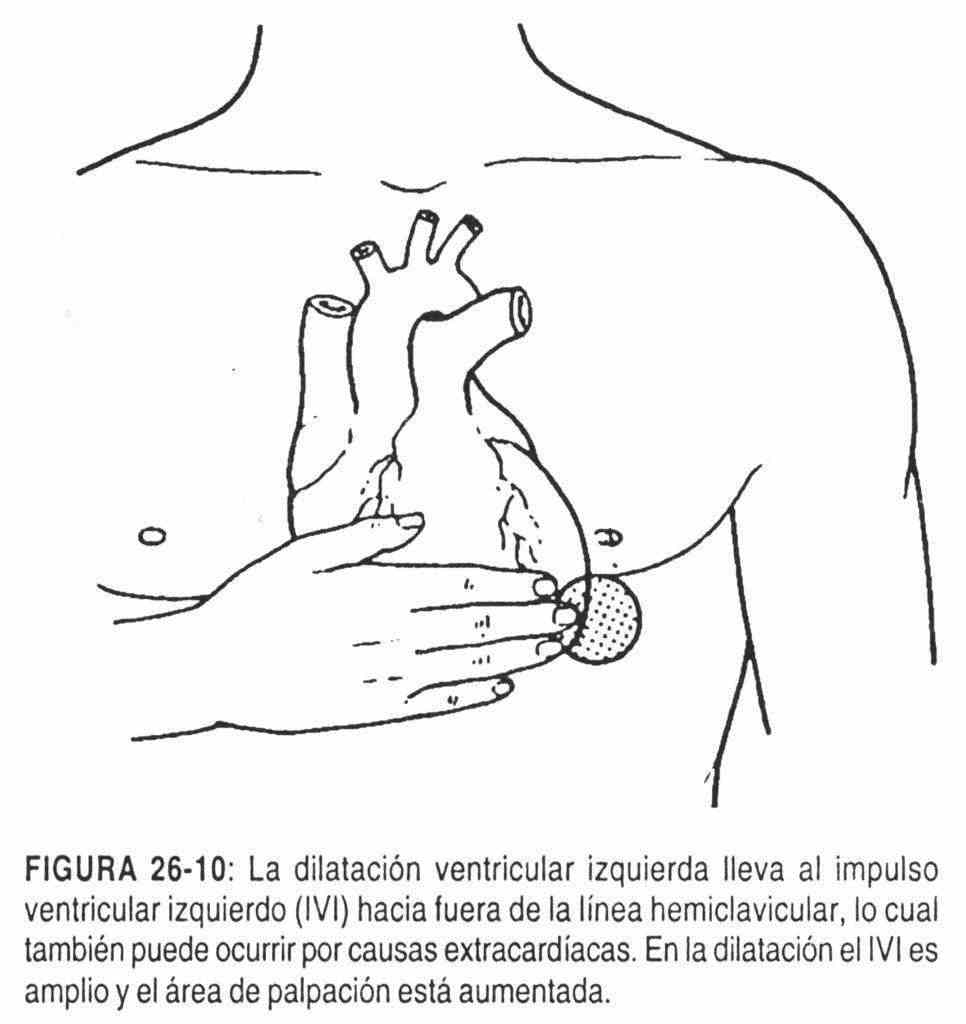
In aortic regurgitation (Figure 26-10), in which the dilation of the left ventricle will predominate over the existing hypertrophy, the left ventricular impulse will be wide, enlarged, but fundamentally displaced to the left
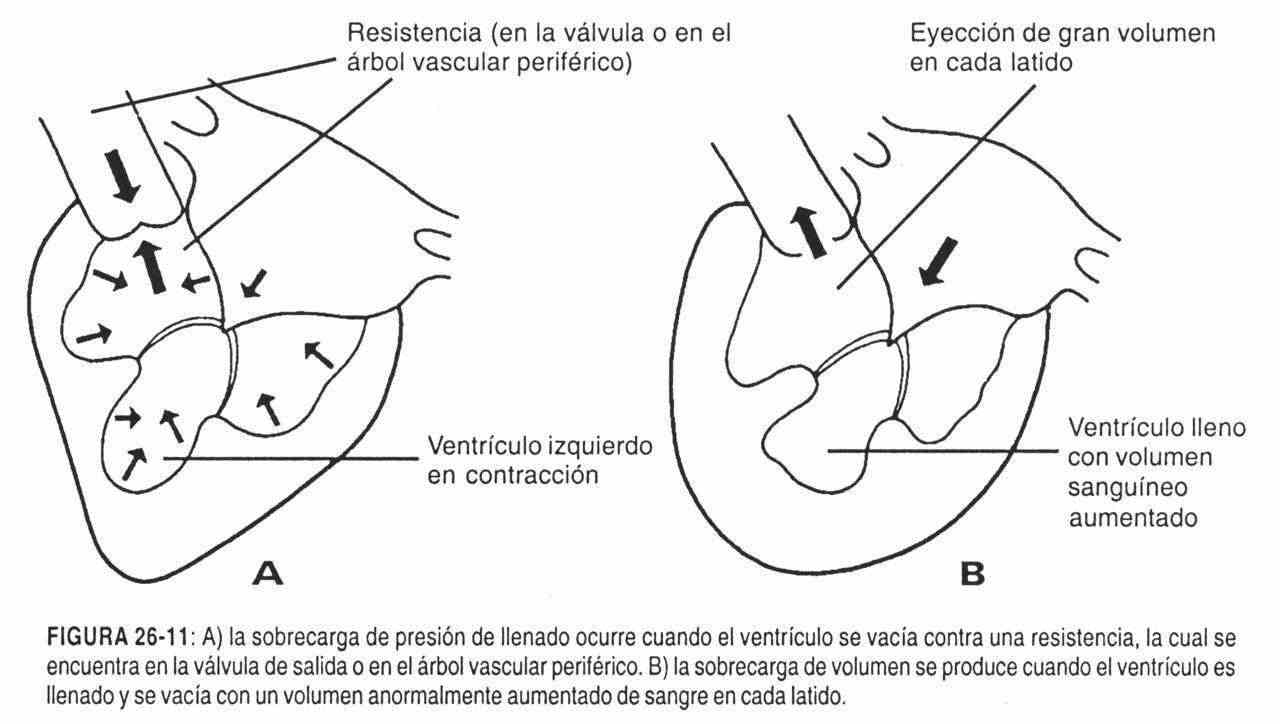
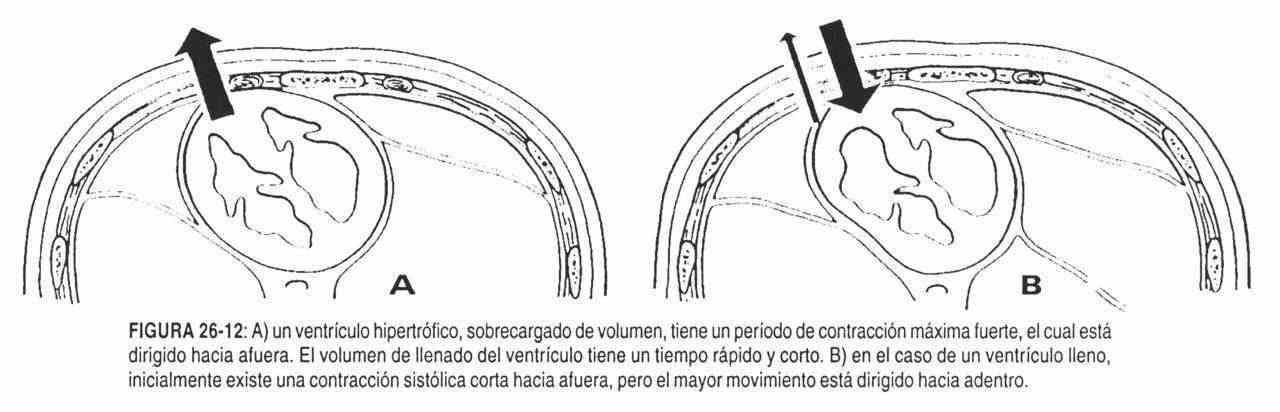
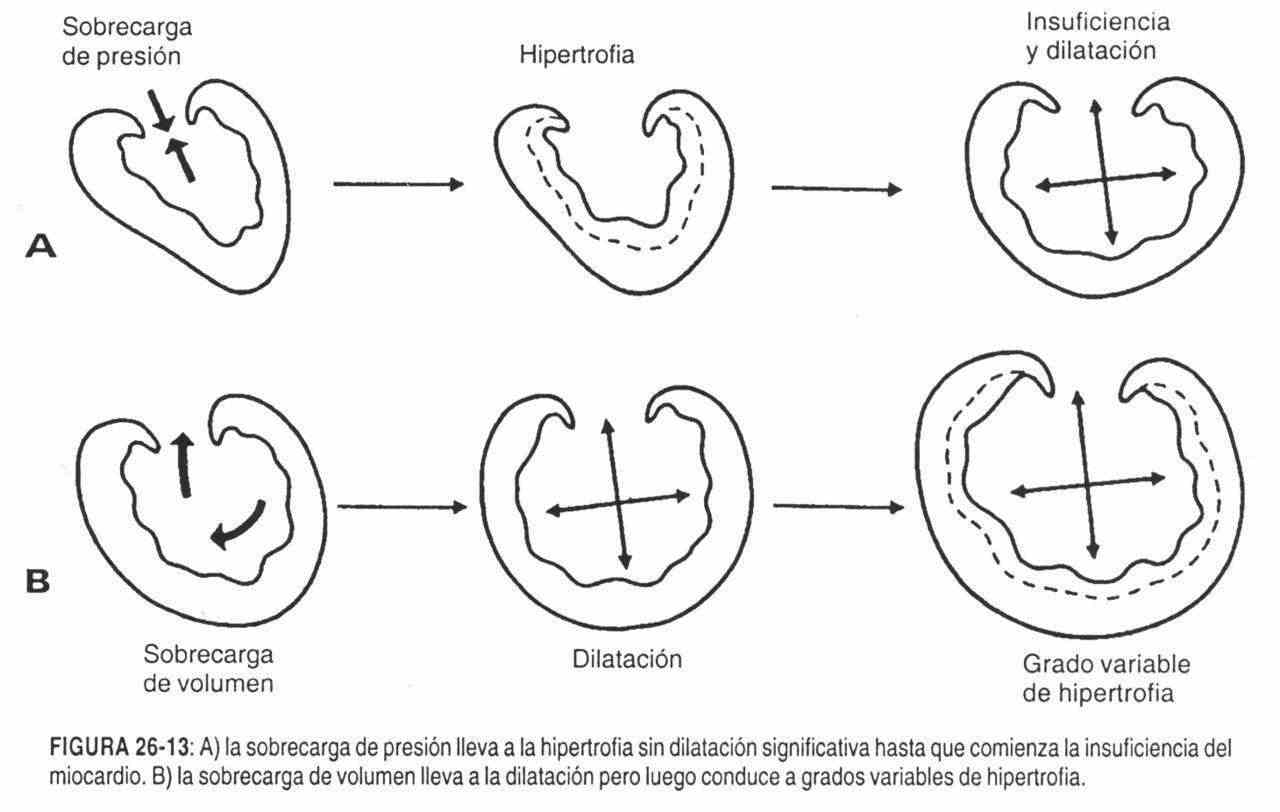
 In the event that the location is displaced with an increase in size, amplitude and rate of ascent, with a normal duration, a chronic overload of the left ventricle should be considered, due to aortic insufficiency with good ventricular function (figures 26-11, 26-12 and 26-13).
In the event that the location is displaced with an increase in size, amplitude and rate of ascent, with a normal duration, a chronic overload of the left ventricle should be considered, due to aortic insufficiency with good ventricular function (figures 26-11, 26-12 and 26-13).
In the case of a dilated left ventricle with marked hypokinesia, the left ventricular impulse will be displaced to the posterior axillary line with a marked decrease in force.
An increase in amplitude can be seen normally in individuals with a flat chest or pectum excavatum. Normally in the left lateral decubitus the left ventricular impulse undergoes a 3 cm displacement towards the anterior axillary line, and it is also normal that the amplitude of the beat is greater. The amplitude increases in cases of fever, anemia, hyperthyroidism and anxiety.
 When the right ventricle is dilated, the ventricular chamber extends to the left, and may even cover the left edge of the heart.
When the right ventricle is dilated, the ventricular chamber extends to the left, and may even cover the left edge of the heart.
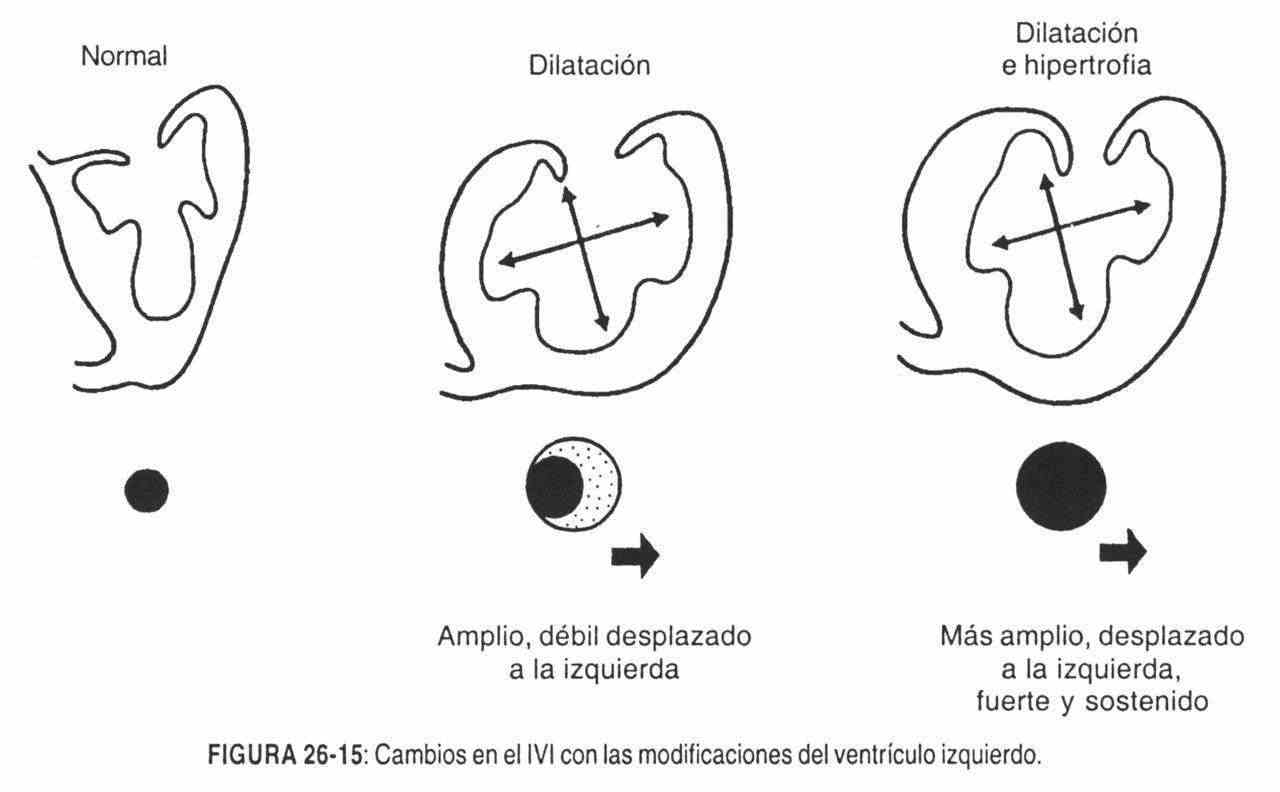
This area is normally occupied by the left ventricle. The right ventricular impulse can appear at the apex and extend beyond the hemiclavicular line and be confused with the left ventricular impulse (Figures 26-14 and 26-15).
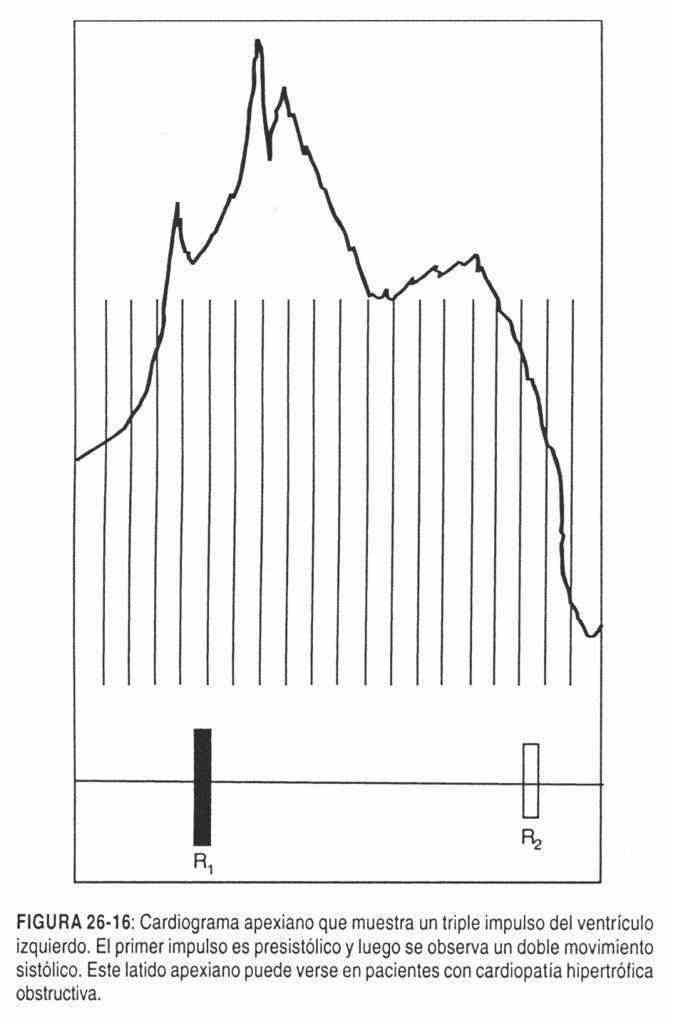
Double boost . In those patients with obstructive hypertrophic heart disease, a double impulse is palpable in systole preceded by a presystolic impulse, which would configure a triple impulse (Figure 26-16). The beats are located in the fifth intercostal space in the hemiclavicular line, without displacement and with an increase in size, strength, duration and speed.
A double or bifid impulse can also be palpated in systole in myocardial infarctions with an aneurysmal area of the left ventricle.
If the third noise is present, a triple impulse will be palpated, and if the third and fourth sounds are present, a quadruple impulse.
Systolic retraction . In constrictive pericarditis, a negative systole beat can be palpated at the level of the left ventricular impulse. Like any negative impulse, it looks better than palpable.
Impulses in diastole (third and fourth sounds) . The third noise can be physiological, that is, normal auscultation in children or in the thorax of adults with special characteristics, but in these cases the sound does not generate a palpable movement.
 The third pathological noise, that is, when the cardiac ejection fraction has dropped to 30% due to hypo or akinesia of the left ventricle, can be palpated when the patient is placed in the appropriate position.
The third pathological noise, that is, when the cardiac ejection fraction has dropped to 30% due to hypo or akinesia of the left ventricle, can be palpated when the patient is placed in the appropriate position.
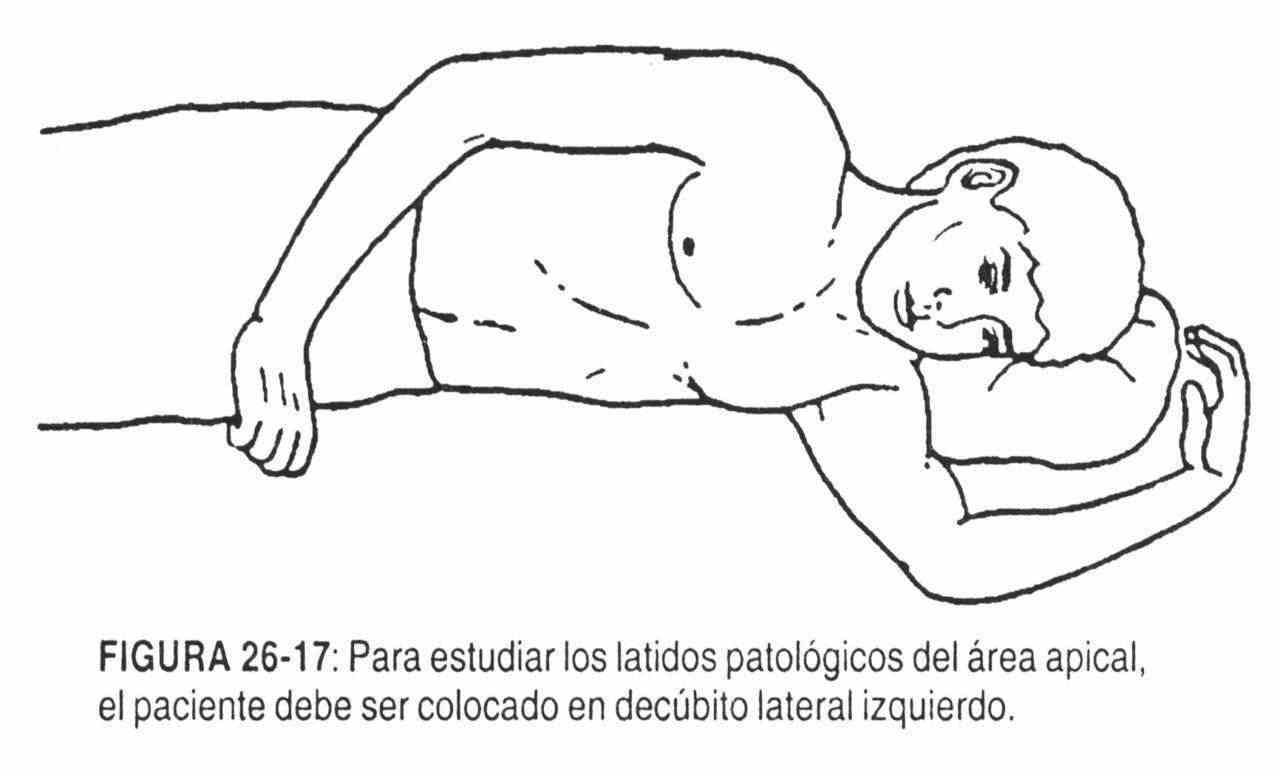
The examinee should be in the left lateral decubitus (Figure 26-17). After searching for the left ventricular impulse with the fingertips of the right hand, it should be defined with one or two fingers of this hand, according to the size of the palpated beat. In this way, it is possible to detect the third noise, or diastolic movement, produced by the entry of blood from the atrium with an increased flow or by a ventricular chamber that is not in a position to receive that input, or perhaps the sum of both phenomena in rapid ventricular filling. This happens in early diastole. This phenomenon can be amplified by requesting the patient for a moment of expiratory apnea. It is also known by the name of "Quran tremor". In left heart failure, in which there is a ventricular gallop due to third noise, As in mitral regurgitation, this movement is palpable. A mid-early diastole beat may also be palpated in constrictive pericarditis. In this case, the pericardium restricts the normal distention of the ventricle in early diastole.
 The fourth noise can be palpated with the same semiological maneuvers that are carried out to locate the third noise. The fourth noise is present in the presystole in pathological conditions, and can be identified if the patient is tachycardiated, causing him to make one or two coughs. Patients suffering from ischemic heart disease due to coronary lesions can develop the fourth noise during chest pain, or during exercise without the need for chest pain, or when asked to elevate the legs.
The fourth noise can be palpated with the same semiological maneuvers that are carried out to locate the third noise. The fourth noise is present in the presystole in pathological conditions, and can be identified if the patient is tachycardiated, causing him to make one or two coughs. Patients suffering from ischemic heart disease due to coronary lesions can develop the fourth noise during chest pain, or during exercise without the need for chest pain, or when asked to elevate the legs.

 When the heart rate increases, it does so at the expense of the diastolic moment, and therefore in tachycardia the third and fourth sounds can be added. Palpation of this beat is even easier when the tachycardia is not very marked. The impulse occurs in the middle of diastole and its amplitude is increased.
When the heart rate increases, it does so at the expense of the diastolic moment, and therefore in tachycardia the third and fourth sounds can be added. Palpation of this beat is even easier when the tachycardia is not very marked. The impulse occurs in the middle of diastole and its amplitude is increased.
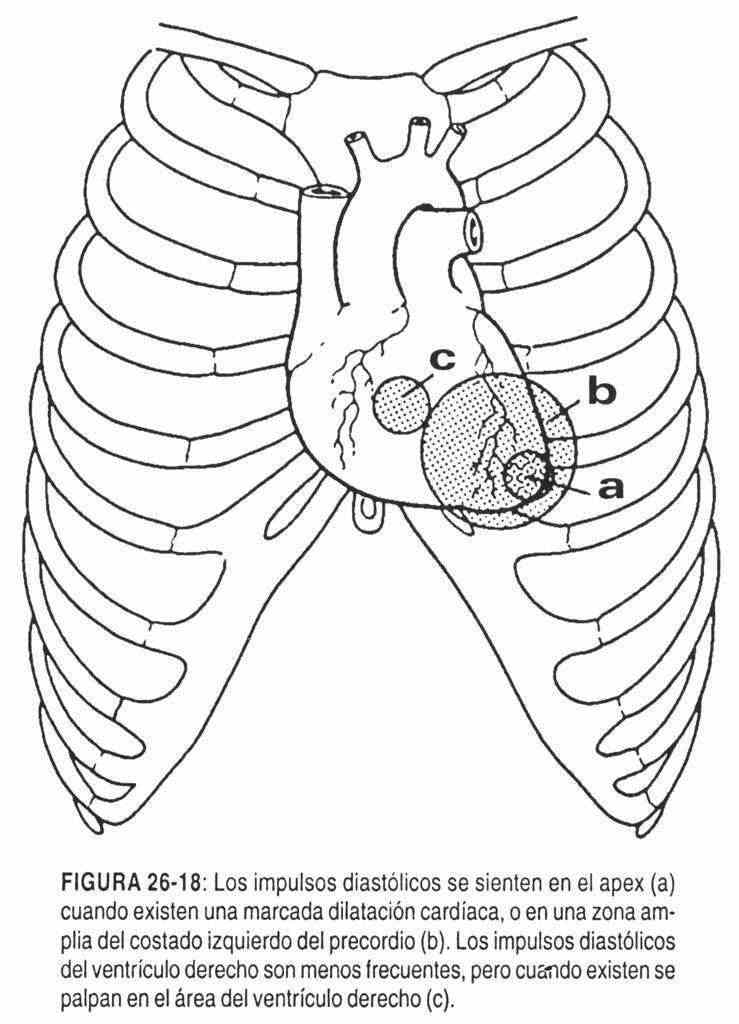
 The palpation areas for diastolic impulses are outlined in Figure 26-18.
The palpation areas for diastolic impulses are outlined in Figure 26-18.
 Palpation of heart sounds.
Palpation of heart sounds.
On certain occasions, and with the patient in the left lateral decubitus position, the first heart sound can be palpated at the level of the left ventricular impulse. For this to be possible, the first noise must be intensified. Mitral stenosis is a good example of this.
The second heart sound, sometimes with a large increase in intensity, can be palpated at the level of the left ventricular impulse. The mere fact of feeling it in this area means that the second noise is increased.
Epigastric area
All those general pictures that are accompanied by cardiac erethism or hyperactivity can present positive systolic movements in the epigastrium. The clearest examples are anemia, fever, and hyperthyroidism.
Aneurysms of the abdominal aorta will be revealed by a clear positive heartbeat, as well as enlarged right heart with hypertrophy.
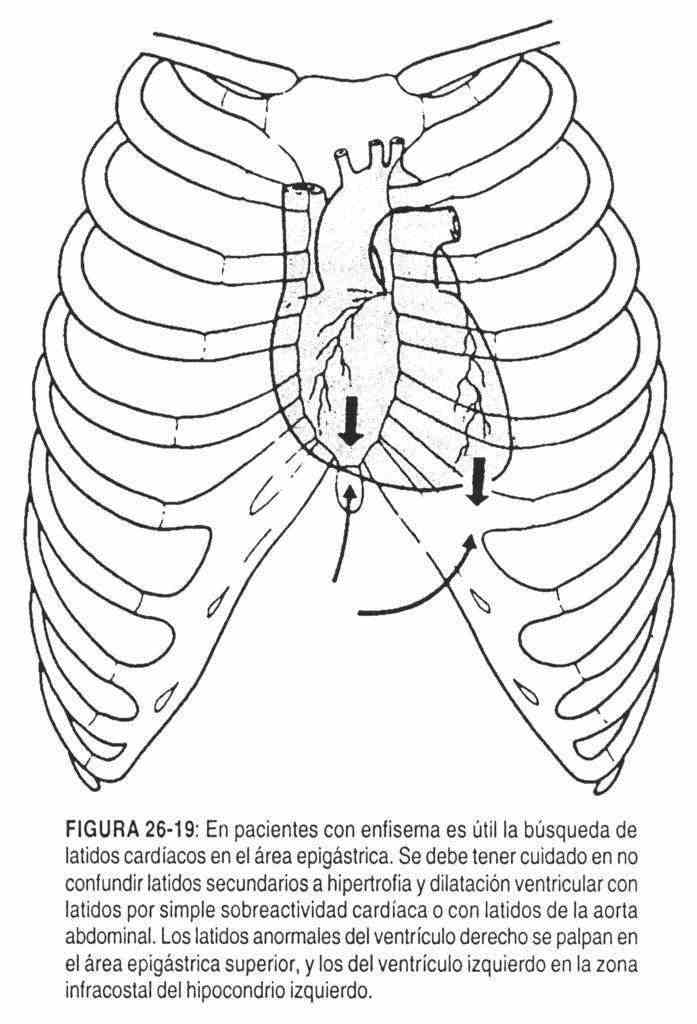
The epigastric area is the indicated site for palpatory examination and cardiac auscultation in patients with marked emphysema or barrel chest. In these pictures, the examiner's hand must sink into the epigastrium below the rib cage, "searching for the heart" (Figure 26-19).
 Palpation of murmurs (thrills)
Palpation of murmurs (thrills)
The auscultatory phenomenon of the murmur can be palpable when it presents a significant amplitude of vibration (from 4/6 to 6/6). The sensation that is perceived is that of a rhythmic vibration with the heart beat.
Murmurs can be found in any area of the precordium and evaluated following the semiological guidelines of location, intensity (amplitude), radiation, and place they occupy in the cardiac cycle.
The search is performed with the patient in the supine position at 45 ° and in the left lateral position when it is necessary to bring the left ventricular wall closer to the precordium.
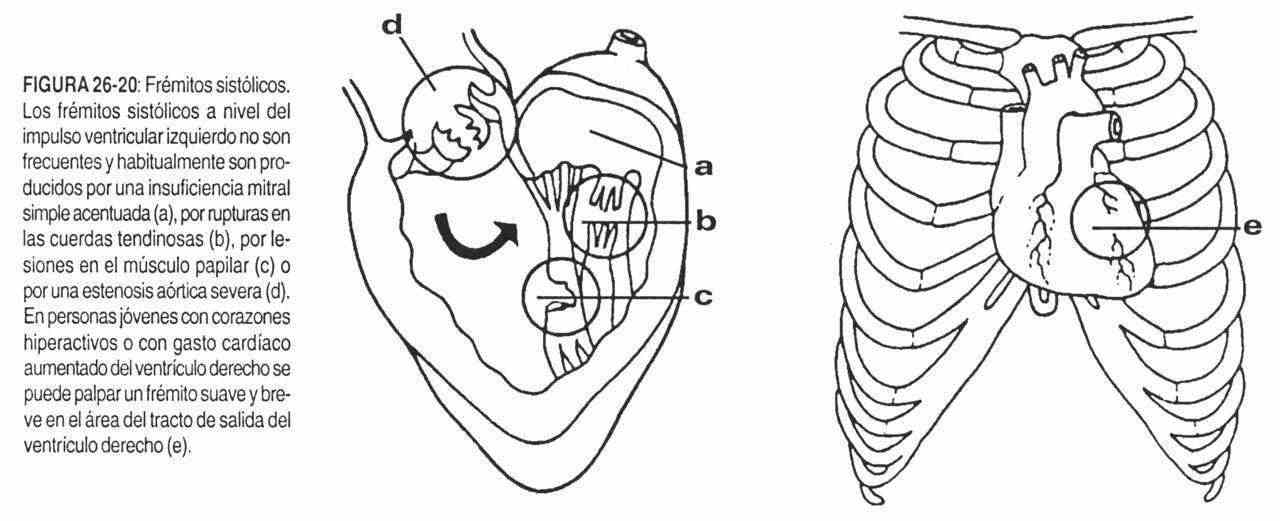 The murmurs of mitral regurgitation and aortic stenosis are clear examples of cardiac lesions with thrills that can be defined by their characteristic semiology (Figure 26-20).
The murmurs of mitral regurgitation and aortic stenosis are clear examples of cardiac lesions with thrills that can be defined by their characteristic semiology (Figure 26-20).
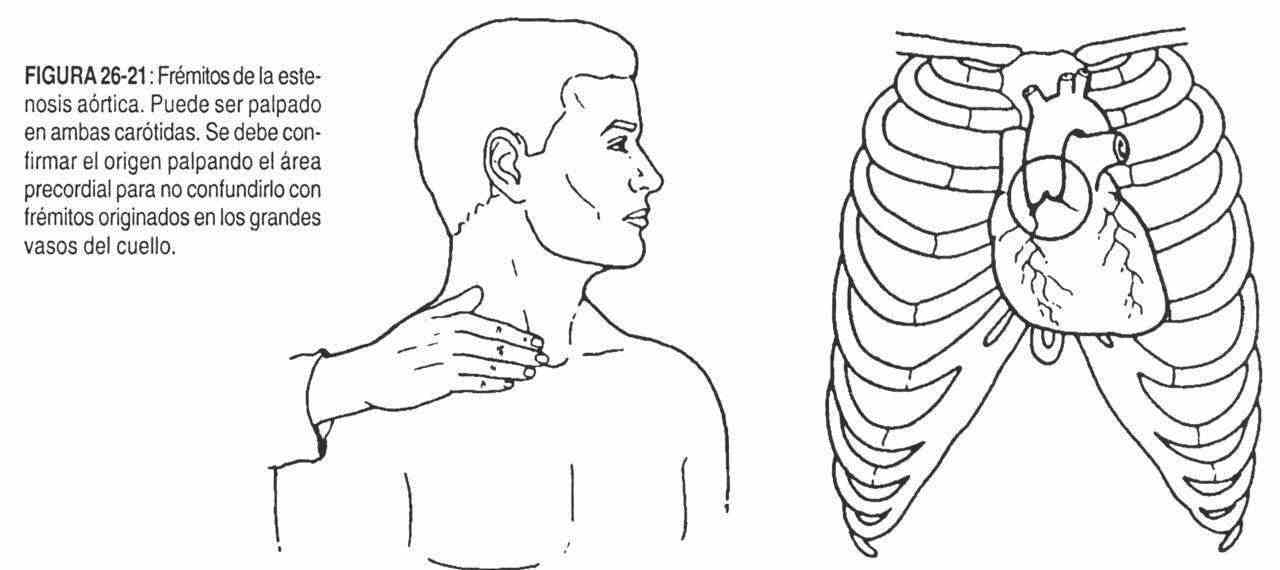
The thrill of mitral regurgitation is systolic, intense, diffuse, in the area of the left ventricle (IVI). In turn, the thrill of aortic stenosis is systolic, wide, in the second right intercostal space, near the sternum, and clearly radiates to the right side of the neck (Figure 26-21).
 The thrill of pulmonary stenosis can be palpated in the left second and third intercostal spaces, near the sternum.
The thrill of pulmonary stenosis can be palpated in the left second and third intercostal spaces, near the sternum.
A systolic thrill at the level of the third, fourth, and fifth intercostal spaces on the left border of the sternum is found in VSD and in tricuspid regurgitation.
The thrill of mitral stenosis is diastolic, located in the area of the left ventricle, with a first palpable noise. The vibrations are of low frequency and therefore are not easily palpable; this is not the case in aortic regurgitation, where they are of high frequency.
Intercostal spaces. In coarctation of the aorta, there is collateral circulation through the intercostal arteries, which can be palpated in these spaces as positive beats synchronous with ventricular systole.
PERCUSSION OF THE PRECORD
Percussion of the heart has fallen into disuse and it could be said that it is not performed in practice. It can provide an idea of the size of the heart, its location, and its borders. In those clinical situations in which inspection and palpation are not helpful, percussion is even less so. Some authors think that it can provide useful information on the presence of pericardial effusions. In practice, however, cardiology has advanced its complementary techniques to a level that makes percussion obsolete.
As already mentioned, inspection and palpation of the precordium must be interrelated, simultaneously and successively. These findings will then be linked to the data obtained from the medical history and will be completed with auscultation, radiology, electrocardiography, echocardiography, etc. Actually, at the patient's bedside, percussion may be helpful in cases where dextrocardia is suspected.
CARDIAC AUSCULTATION
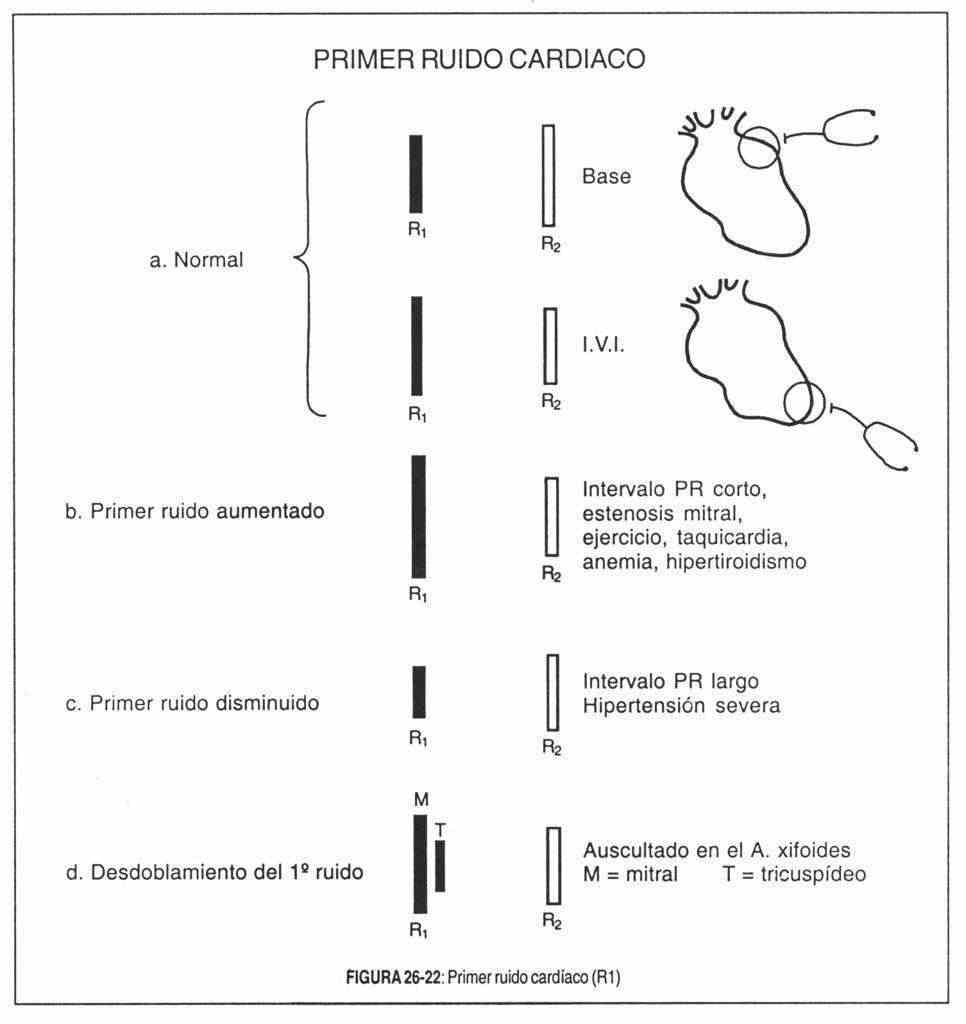 First noise (Rl)
First noise (Rl)
Alterations in the first sound (Figure 26-22) are evidenced by changes in its intensity and by the splitting of its mitral and tricuspid components, a split that in some circumstances has pathological significance. The timbre of the first noise is less important than its intensity.
Modifications of intensity . The factors that modify the intensity of the first sound can be of cardiac or extracardiac origin. Extracardiac factors generally modify the first and second sounds together. Cardiac factors, on the other hand, mainly modify one of the two.
Extracardiac factors that modify the intensity of the first noise . These include:
1) Chest wall thickness: The thicker the wall, the more the sound will be weakened by damping and reflection. In a thick-walled individual, the noises will be more muffled than in a thin-walled person or a child. The same occurs in obese individuals and in women with large breasts. In these cases, the noises are best heard in a sitting position; 2) emphysema: this lung condition can completely shut off heart sounds; 3) pericardial effusion: it can weaken heart sounds, both by increasing the distance between the heart and the wall and by causing new sound reflections.
Cardiac factors that modify the intensity of the first sound. The intensity or amplitude of the first sound depends on: 1) the position of the atrioventricular valves at the onset of ventricular systole; this factor is the most important; 2) the valve structure; 3) the degree of tension and pressure developed in the ventricular chambers. Therefore, there will be a loud first noise when the valves are wide open at the time ventricular systole begins and for this reason they have a long way to go to close. This occurs due to the prolongation of the passage of current from the atria to the ventricles in two circumstances: when the PR space is shortened, and when the atrial pressure remains elevated at the end of diastole (mitral and tricuspid stenosis, hyperkinetic circulatory states, short circuits from left to right,
When the PR space of atrioventicular conduction is short (0.08-0.12 sec), the rush of blood to the ventricles by atrial contraction opens the atrioventricular valves wide immediately before ventricular contraction. Hence the first loud noise characteristic of preexcitation syndrome (Wolff-Parkinson-White) and the first cannon shot of complete AV block. In the latter pathology, the variable atrioventricular relationships originate a first changing tone, which is maximum, in a cannonball when the atrial contraction precedes the ventricular by 0.10-0.12 seconds, or overlaps.
In mitral stenosis, the atrial pressure remains elevated until the end of diastole, keeping the leaflets widely open until the last moment, thus causing the first loud noise. The intraventricular pressure also contributes to this, which must reach a higher level before closing the valve, so the closing pressure will be higher and the valve will close more abruptly. Also in mitral stenosis, the first loud noise will depend on the valve structure: it is very possible that the fusion of the mitral valve commissures and the shortening of the chordae tendineae make the valve apparatus vibrate in toto and that the first loud noise , with a clicking sound, be the systolic counterpart of the opening click, configuring a true closing click,
For this, the valve apparatus must retain a certain degree of flexibility because when the leaflets harden and immobilize, as in calcified mitral stenosis, the first loud noise and its clicking character disappear. In mitral stenosis, the first accentuated noise - together with the presystolic murmur - is the precocious sign par excellence, even in mild cases, when the valve diameter is around 2 cm2 (the normal orifice measures 4 to 6 cm2) and the patient is still completely asymptomatic.
Increased ventricular filling, with increased atrioventricular flow from left to right short circuits and hyperkinetic circulatory states (hyperthyroidism, exercise, fever, anemia) increases the intensity of the first noise. In the latter, the accentuation of the first sound is made at the expense of both components, mitral and tricuspid, although the mitral is usually the dominant one. On the contrary, in communications from left to right, and because the overload is elective, the first noise results from the accentuation of the mitral component (patent ductus, ventricular septal defect) or tricuspid (atrial septal defect).
The first faint noise occurs when at the time of ventricular systole the atrioventricular leaflets are floating almost in opposition. When the PR space exceeds 0.22 seconds (first degree AV block) the first noise can, for this reason, be extremely weak. This finding is important: a markedly diminished noise in the apical region makes it possible to suspect the existence of a prolonged or borderline PR space. Active rheumatic carditis prolongs this atrioventricular conduction PR time and hence its first dull tone. In shock states the same situation occurs due to a decrease in venous filling pressure, and the same occurs in constrictive pericarditis. The first faint sound of calcific mitral stenosis and mitral regurgitation has a different origin,
Doubling of the first noise . Let us remember that the first sound can normally be unfolded in an area of the precordium between the mitral and tricuspid foci, due to the normal asynchronism between the closures of both atrioventricular valves. In the mitral focus, the most intense mitral component is auscultated, masking the tricuspid.
In the tricuspid focus, the reverse occurs, although the mitral component is frequently auscultated. Between the two foci there is an area where the two components are clearly auscultated as an obvious split. Its clinical interest lies in the differentiation with the fourth noise or atrial gallop, with the systolic ejection sound and with the atrial presystolic murmur.
The ejection sound is better heard at the base, the unfolding of the first noise is not usually transmitted to the base. The atrial gallop is low in pitch, and the presystolic murmur is greatest at the tip, increases after exercise, and is accompanied by other signs of mitral stenosis, such as loud first sound, opening click, and diastolic rumbling.
When the unfolding of the first sound is extensive and is heard in both the mitral and tricuspid foci, it is usually pathological. It is always found in delays in the tricuspid component, such as complete right bundle branch block (where there is also wide splitting of the second sound in the lung area), in atrial septal defect, in Ebstein's disease and in tricuspid stenosis .
If the mitral component is delayed, surpassing the tricuspid, the so-called paradoxical or inverted doubling of the first sound takes place, because its components are not only unfold but also chronologically inverted. In severe mitral stenosis, the mitral component is often delayed because the left intraventricular pressure must rise more than normal before exceeding the high pressure in the left atrium and thus closing the valve. When this delay is significant, the valve closure order can be reversed. This finding is a phonocardiographic sign, although it can sometimes be recognized by auscultation.
Second noise (R2)
Careful clinical evaluation of the second heart sound is a valuable routine test for the diagnosis of heart disease.
It has already been said that there is a physiological splitting in the lung area, during inspiration, in most children, adolescents and young adults, and rare in subjects over fifty years of age.
In the analysis of the abnormalities of the second noise, the following should be studied:
- pathological unfolding
- reverse splitting (paradoxical)
- second single noise
- accentuation of second noise
- decrease of second noise
It is important to remember that the physician should be alert to the possibility of heart disease when listening to a wide splitting of the second sound on expiration, particularly after the age of fifty. In this sense, it is useful to evaluate the effect of sitting position on audible expiratory unfolding. In almost all adults with heart disease who present this finding in the decubitus position, it will persist in the sitting position. It should be taken into account that 17% of normal individuals between the ages of twenty-one and thirty have audible expiratory splitting and only 4% of normal individuals over fifty years of age present it. In normal children and adolescents with this sign, it can persist in a sitting position.
Pathological unfolding of the second noise . Expiratory unfolding may be due to early closure of the aortic valve or, more commonly, to delayed closure of the pulmonary sigmoid.
A delayed second lung sound can have three types of causes:
- electrical delay in right ventricular activation, as in complete right bundle branch block;
- dynamic delay due to prolongation of right ventricular expulsion, due to an overload of the volume of the right heart (atrial septal defect, abnormal pulmonary vein drainage);
- mechanical delay, due to increased resistance to right ventricular emptying (pulmonary stenosis).
In contrast, an early second aortic sound may be due to a shortened left ventricular ejection, as in mitral regurgitation or ventricular septal defect, or a decrease in left ventricular stroke volume.
Whatever the case, an audible expiratory splitting of the second sound, which persists in the upright position in the adult, should suggest heart disease. The causes of audible expiratory splitting of the second sound are summarized in Table 26-1.
|
Table 26-1 |
|
In complete right bundle branch block, there is wide splitting of the first and second sounds, and as the cardiac dynamics are unchanged, splitting of the second sound widens on inspiration.
Fixed doubling of the second sound is the most important clinical sign of atrial septal defect (Figure 26-23). It is "fixed" because the separation of its two components (A2 and P2) does not vary in both respiratory phases for more than 0.015 seconds. It also does not vary with standing or with the Valsalva maneuver. Shafter's studies explain that during inspiration, the filling of the right ventricle increases, although to a lesser degree than in normal subjects, and the pulmonary component slightly exceeds, but the aortic component instead of anticipating as in normal conditions, is also it delays an interval similar to that of the lung, leaving the A2-P2 interval practically the same as in expiration.
In pure pulmonary stenosis, the second sound is usually doubled due to the delay in pulmonary valve closure, caused in turn by the lengthening of the expulsive period of the right ventricle. However, when the stenosis is very severe, the pulmonary component is inaudible (right ventricular pressure greater than 130 mm Hg), due to the drop in flow pressure in the pulmonary circulation. In any case, and with the exception of this last circumstance, it is useful to remember that the A2-P2 interval maintains, in pulmonary stenosis, a quantitative relationship with the systolic pressure of the right ventricle. Thus, in mild and moderate stenoses (from 30 to 80 mm Hg) the expiratory split will be 0.04 to 0.08 seconds, and greater in severe obstructions. The A2-P2 interval increases on inspiration and after exertion, especially in light and moderate cases; in severe cases it can be "fixed".
Patients with medium or large VSDs may have audible expiratory doubling of the second sound, due to the prolongation of the right ventricular ejection time and the expulsive shortening of the left ventricle.
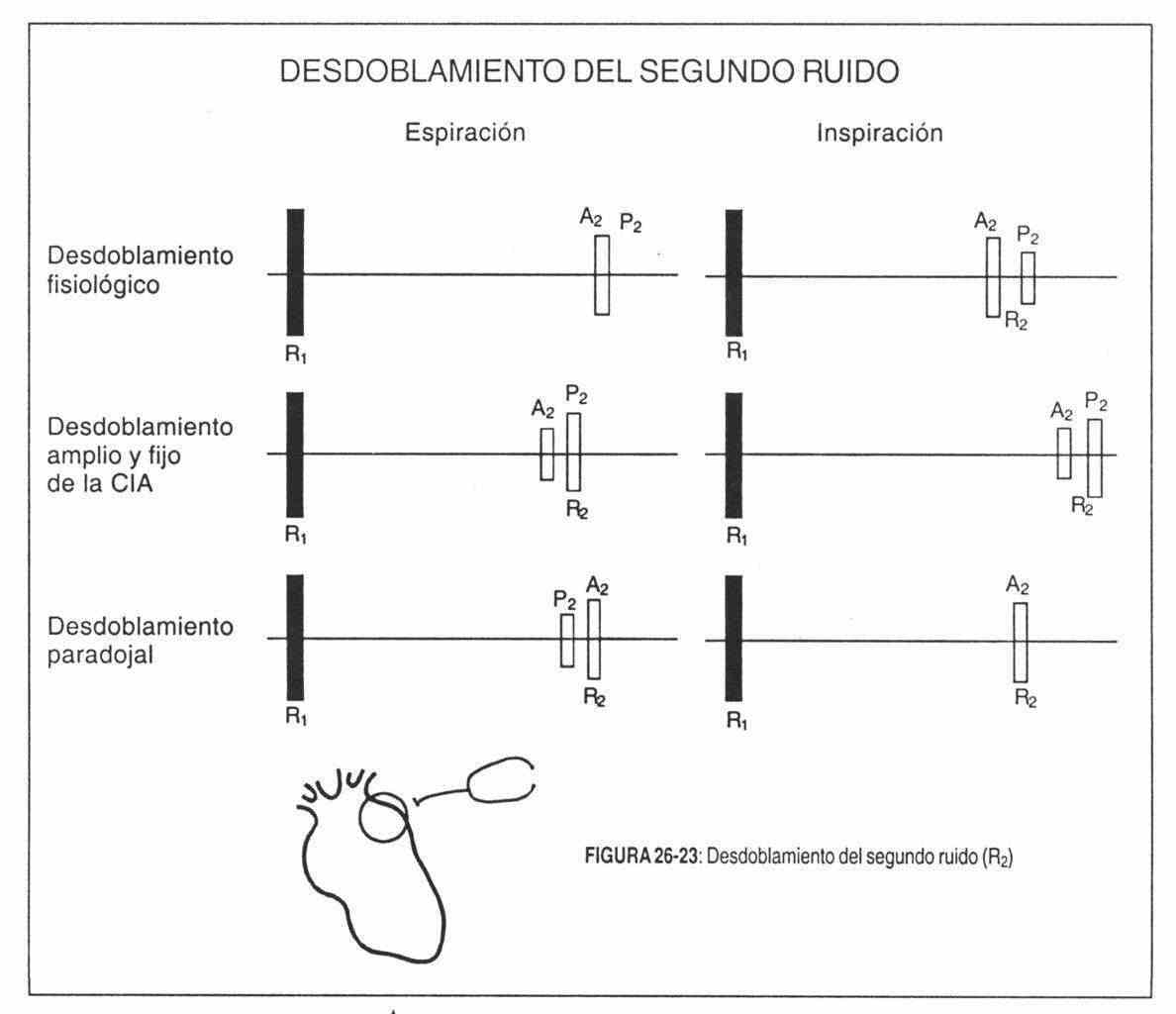 Inverted (paradoxical) doubling of the second noise (Table 26-2). The pulmonary component precedes the aortic, which is far behind. However, since the pulmonary component is normally delayed during inspiration, the splitting is closed or attenuated during inspiration and widens on expiration. This is its fundamental characteristic (Figure 26-23). The delay of the aortic component is also of three types: electrical, mechanical and dynamic.
Inverted (paradoxical) doubling of the second noise (Table 26-2). The pulmonary component precedes the aortic, which is far behind. However, since the pulmonary component is normally delayed during inspiration, the splitting is closed or attenuated during inspiration and widens on expiration. This is its fundamental characteristic (Figure 26-23). The delay of the aortic component is also of three types: electrical, mechanical and dynamic.
The most common cause of reverse doubling of the second sound is complete left bundle branch block, which causes electrical delay in aortic closure. This unfolding is found in 84% of patients with left bundle branch block.
|
Table 26-2 |
|
More difficult to detect clinically is the paradoxical splitting in aortic stenosis, where the left ventricular mechanical systole is prolonged to overcome the obstruction, thus delaying the aortic component of the second sound (mechanical delay).
In major patent ductus, the exclusive left ventricular period is prolonged by its volumetric overload, thus delaying the aortic component (dynamic delay). In addition, it can occasionally be found during angina attacks, after a myocardial infarction, or in cardiomyopathies.
Unique second noise(table 26-3). In certain circumstances, the second heart sound is unique. This is usually observed in tetralogy of Fallot, due to the absence of the pulmonary component; here the pulmonary flow is so low that there is no second lung sound, which only appears after surgical correction of the malformation. In patients with severe pulmonary valve stenosis, the highly delayed lung noise may be inaudible. The second noise is usually unique in tricuspid atresia. In patients with a trunk or pseudo-trunk arteriosus, in whom there is only one arterial valve, the second noise is also unique. Likewise, this tone may be unique in severe aortic stenosis; Given the expulsive lengthening of the left ventricle, the aortic component is delayed and may coincide with the pulmonary component. It can also be seen in left bundle branch block. But in these last two pathologies it is more common to find an inverted doubling of the second noise.
Changes in intensity of the second noise . The factors that can produce changes in the intensity of the second sound can be classified as extracardiac and cardiac. The extracardiac factors are similar to those that influenced the first sound, and generally affect both tones at the same time.
|
Table 26-3 |
|
The cardiac factors that affect the intensity of the second sound are: 1) systemic and pulmonary diastolic pressure; 2) pathological changes in valves and great vessels.
Accentuation of the second noise . The intensity of the noise produced by sigmoid valve closure increases with increasing closure pressure in the vessels.
Thus, the second loud tone is a consistent finding in pulmonary and systemic arterial hypertension (Figure 26-24).
In pulmonary arterial hypertension, accentuation of the easily palpable pulmonary component ("pulmonary closure shock") is found in any of the many conditions that generate pulmonary hypertension. The pulmonary component is transmitted to the tip, the only circumstance in which the second mitral noise is formed by the pulmonary component.
In severe pulmonary hypertensions of any origin, it is common to observe the so-called "pulmonary complex" of Chávez or "left parasternal syndrome", which is constituted by: a) systolic rise in the pulmonary area; b) palpable pulmonary valve closure shock, and c) zone of dullness to percussion, 1.5 to 5 cm wide, in the second left intercostal space next to the sternum. These signs are highly suggestive of a significant dilation of the pulmonary artery associated with hypertension of said artery.
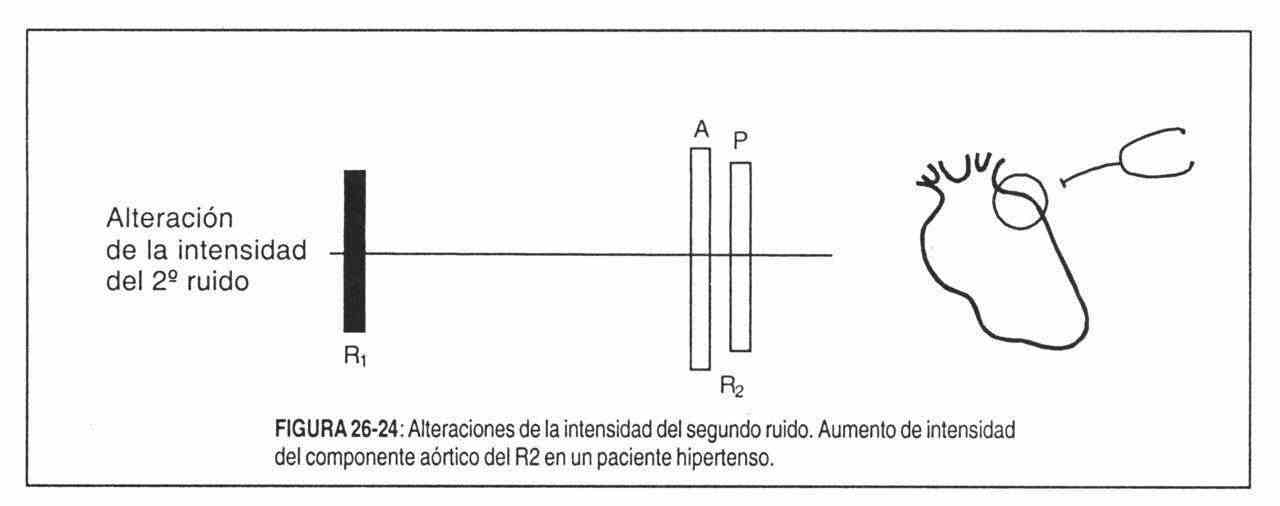 The aortic component of the second tone is accentuated in systemic arterial hypertension, aortic sclerosis, and is frankly strong in some isolated patient with syphilitic aortitis or ascending aortic atherosclerosis.
The aortic component of the second tone is accentuated in systemic arterial hypertension, aortic sclerosis, and is frankly strong in some isolated patient with syphilitic aortitis or ascending aortic atherosclerosis.
Decrease in second noise . The pulmonary component of the second sound is frankly diminished in moderate pulmonary stenosis (in severe cases it disappears) or when there is a decrease in pulmonary resistance.
The aortic component, in turn, is diminished in severe or calcific aortic stenosis and in severe aortic regurgitation.
Both components of the second sound are weak in states of shock, cardiac tamponade (in both cases due to arterial hypotension) and in paroxysmal tachyarrhythmias.
Abnormal aggregate noises. Third and fourth pathological noises. Gallop rhythm.
The criterion that distinguishes normal third and fourth sounds from pathological ones is still practical in many cases. Thus, the normal fourth noise, which is only heard exceptionally in children and adolescents, can be considered pathological in adults.
From a semiological point of view, the meaning of a three-beat rhythm and a gallop rhythm must be defined.
The three-beat rhythm indicates the presence of an added diastolic noise (third or fourth), separated from the normal noises by a time long enough to change the normal two-beat rhythm to a three-beat one.
The gallop rhythm, a very descriptive French expression, presupposes the existence of a three-beat rhythm with tachycardia, which originates a typical cadence, similar to the gallop of a horse.
When the rate is normal or slow, the cadence of the canter rhythm is lost, but this does not detract from the added noise. In other words, the important thing is the presence of the third or fourth noise, whatever the heart rate.
to. Atrial gallop or presystolic gallop or pathological fourth noise . It consists of the addition of a faint, muffled, and dull presystolic extratone (fourth sound) to normal sounds, resulting in a characteristic three-beat rhythm (Figure 26-25). With a high frequency it is the gallop par excellence, and the most common to find.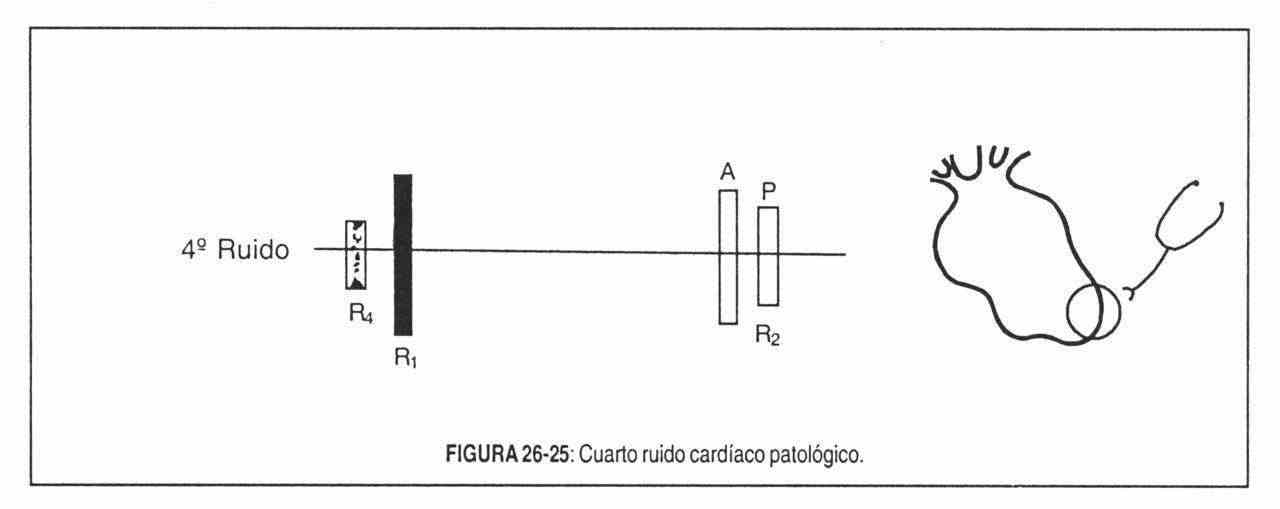
Like all three-beat rhythms, it is a ventricular filling sound produced in this case by the sudden distention of the ventricle caused by atrial contraction. There is an abnormal elevation of end-diastolic pressure and decreased ventricular compliance, either due to hypertrophy or fibrosis. Atrial gallop, therefore, represents a violent or forced opening of the atrioventricular valve caused by intense atrial contraction against a stiffer ventricle with elevated internal pressure.
The atrial gallop is the pathological counterpart of the fourth noise, with the particularity that the normal, easily recordable fourth noise is almost always inaudible. It is often palpable, and since it is of a very low frequency, it should be auscultated by gently applying the bell of the stethoscope to the skin. It is heard electively in the region of the tip and, more frequently, next to the xiphoid appendix, even in the left gallop, perhaps because in this region the first sound is weaker.
Deciding whether a presystolic canter is left or right rests more on clinical reasons than on auscultatory data, except that the canter is accentuated on inspiration, in which case its origin is right. As is logical, it disappears in atrial fibrillation.
The left atrial gallop -or the fourth sound- is very common in systemic arterial hypertension with left ventricular hypertrophy, in acute myocardial infarction and in myocardipatias.
It is rarer in aortic valve disease.
Right atrial gallop is heard in marked pulmonary hypertension, right heart failure, severe pulmonary stenosis, etc. It is accompanied by a sharp, sharp, giant wave in the venous pulse.
b. Third pathological noise or ventricular early diastolic gallop . In subjects over 40 years of age, the third noise should be considered, in principle, "pathological" ("ventricular gallop") until careful investigation shows otherwise.
The early diastolic gallop is produced by the addition of a third pathological noise that creates a three-beat rhythm (Figure 26-26). The cause of its production is accepted as the vibrations produced by the sudden distension of the ventricular walls during the burst of blood at the end of the period of rapid filling. It is a sign of real ventricular failure and not imminent, as would be the atrial gallop.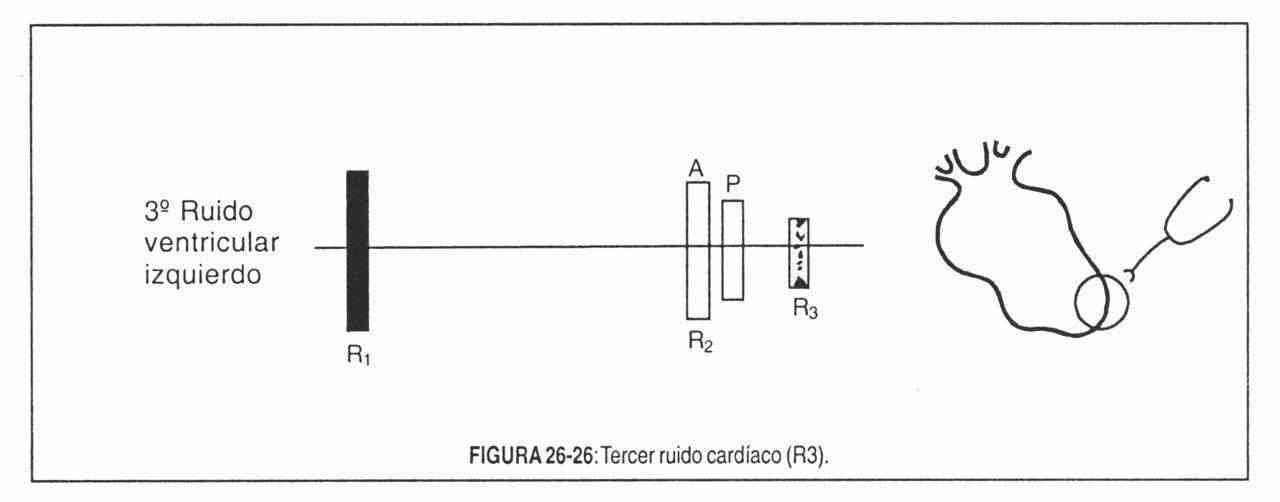
The third pathological noise is muffled and of low frequency, which is why it is better perceived with the bell, in a restricted area of the tip in the left canter and in the tricuspid focus on the right. It is usually accompanied by other manifestations of ventricular claudication, among which, of course, is tachycardia.
The third noise usually appears in hyperkinetic syndromes such as: fever, anemia, etc .; in atrioventricular hyperflow such as mitral regurgitation, ventricular and atrial septal defects, the ductus, etc., and in ventricular insufficiency, which is the most important. Whatever the cause, ventricular failure should be considered whenever a proto-diastolic gallop is encountered.
c. Gallop of sum . When the period of ventricular diastole is shortened by the effect of tachycardia, the third and fourth sounds may merge, generating a summation gallop. In this case, the intensity of the added noise may be greater than that of the first or second noise (Figure 26-27).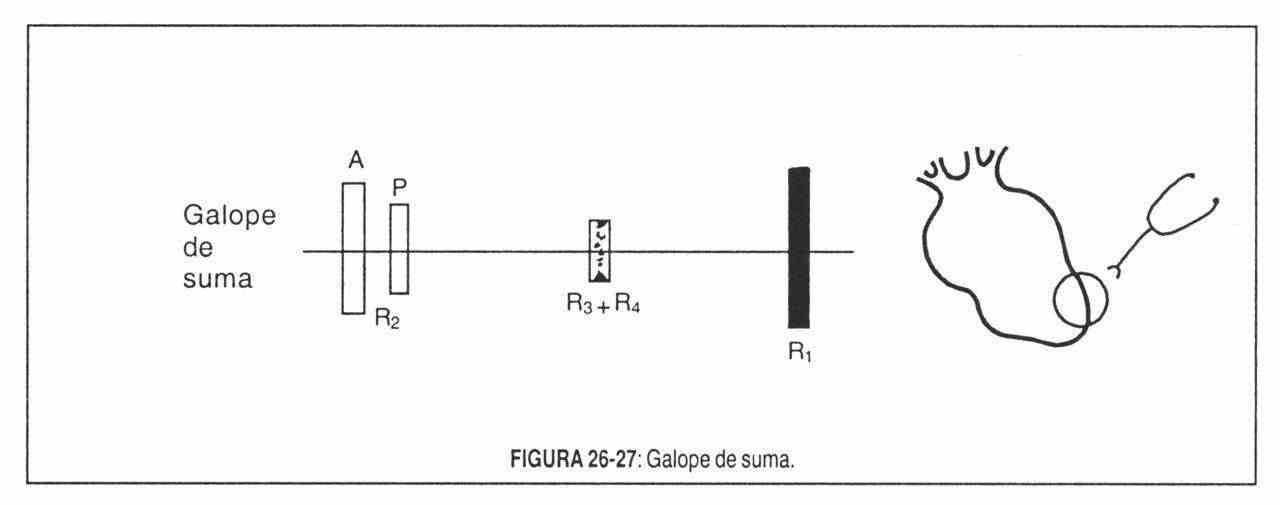
ABNORMAL DIASTOLIC NOISES
Mitral opening snap. It is actually a reinforcement of a subaudible component of the terminal segment of the second noise. It occurs between the second and third sounds, at the end of the isometric relaxation period (Figure 26-28), when elevated atrial pressure abruptly projects the nucleus leaflets into the ventricular chamber. To understand this, it must be remembered that the mitral valve, in mitral stenosis, is a diaphragm perforated in the center, with the commissures fused and therefore, unable to open normally. In addition, a certain degree of flexibility is necessary for the snap to appear, since it does not appear when the stenotic valve is highly calcified. Clicking or popping is possibly at the beginning of valve movement, before the valve opens widely,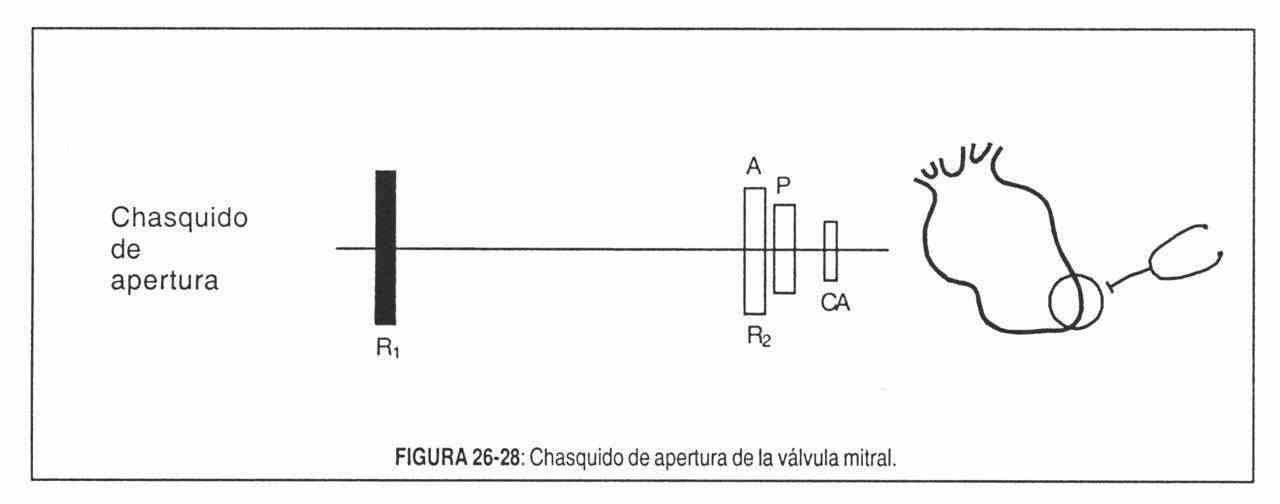
The opening click is a short, high-pitched sound with a high pitch and dry timbre, its intensity ranging from barely audible to that of the first or second noise. It is best heard in the left lateral decubitus position, slightly within the left ventricular impulse or in the left third and fourth intercostal spaces adjacent to the sternum. If it is intense, it can be auscultated in the entire precordium.
Although persistent ductus and ventricular septal defect can produce weak opening clicks due to hyperflow, the presence of this acoustic phenomenon means, almost as a rule, the existence of mitral stenosis, which can be minimal or very severe, since the click does not indicate the hemodynamic severity of the injury.
The interval between the second sound and the click is, on the other hand, in relation to the atrial pressure and, therefore, is an index of the severity of the stenosis. The more closed the stenosis, the higher the left atrial pressure and the earlier the clicking will appear.
The tricuspid opening snap is very similar to the mitral, but higher in pitch, and less loud. It notably increases in inspiration and behaves like a right in the Valsalva maneuver. It is found in tricuspid stenosis, Ebstein's disease, atrial septal defect, and abnormal venous return, the latter due to increased flow through the tricuspid.
ABNORMAL SYSTOLIC NOISES
Clicks. Ejection sounds . Clicks are sharp, short, high-frequency, loud sounds of the popping type and easy audibility that occur in systole. The main characteristic is its sharpness, which with its intensity gives it its own characteristic timbre.
They can be divided into:
- Protosystolic or ejection clicks. Leatham called them "early systolic sounds", and Lian in 1937 called them "pops." They can be aortic or pulmonary.
- Meso and end-systolic or non-ejection clicks. They are generally of mitral and / or tricuspid origin.
Thus, and according to the moment of appearance in systole, clicks are usually divided into: protosystolic or ejection, as they correspond to the beginning of the rapid ejection period, and mesothelial or non-ejection, which correspond to the period of reduced ejection and that they can present as single or multiple auditory phenomena; they are called "non-expulsive" because, although they occur during systolic ejection, they do not depend on it but on other factors, which will be analyzed later.
Perloff, for educational purposes, prefers to call "ejection sounds" to proto-systolic clicks, caused by the sudden burst of blood flow into the dilated aorta or lung, and reserves the name of clicks for "non-expulsive."
1. Ejection sounds or clicks . They are located at the beginning of ventricular expulsion or protosystole, and originate in the sigmoid valves (valve sound) or in the distended vascular wall.
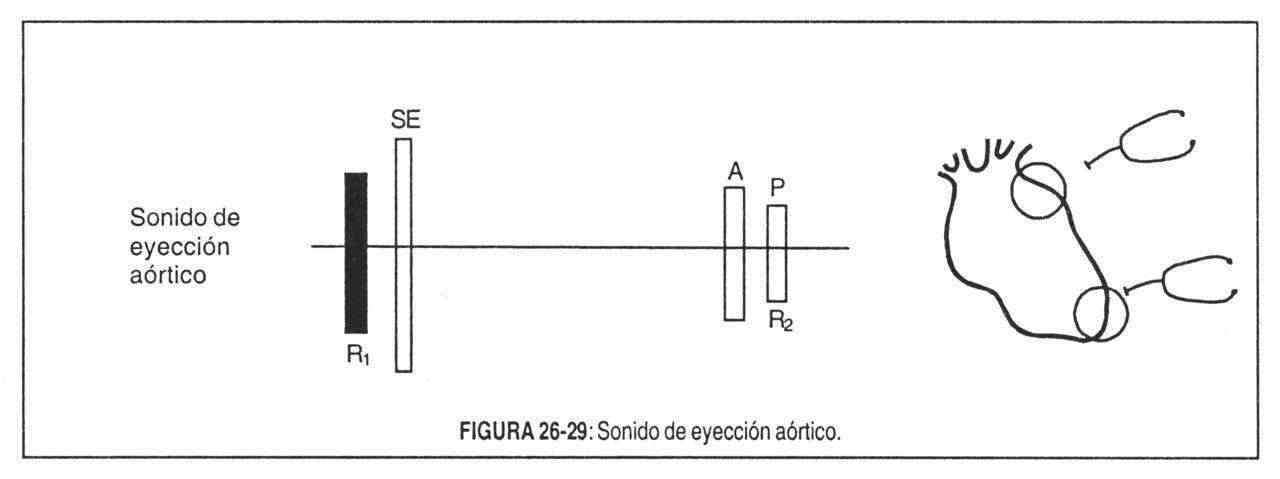 The aortic ejection sound (Figure 26-29) appears in cases of aortic dilatation (aortic stenosis, aortic regurgitation, aneurysms of the ascending aorta, aortic coarctation) and is perceived in the third left parastemal intercostal space, in the aortic focus, in the tricuspid focus and at the apex.
The aortic ejection sound (Figure 26-29) appears in cases of aortic dilatation (aortic stenosis, aortic regurgitation, aneurysms of the ascending aorta, aortic coarctation) and is perceived in the third left parastemal intercostal space, in the aortic focus, in the tricuspid focus and at the apex.
If there is aortic stenosis, the appearance of this systolic sound or click will identify the lesion as valvular, since it is exceptional that it exists in the presence of supra or subvalvular obstructions. In addition, it reports that the stenosis is moderate or mild, and that the valve is mobile. In marked obstructive lesions, expulsion is slow, scant, and does not distend the aorta suddenly.
Aortic genesis is also the click of pulmonary atresia, tetralogy of Fallot, aortic dissection, aortic atheroma, transposition of the great vessels and the trunk arteriosus.
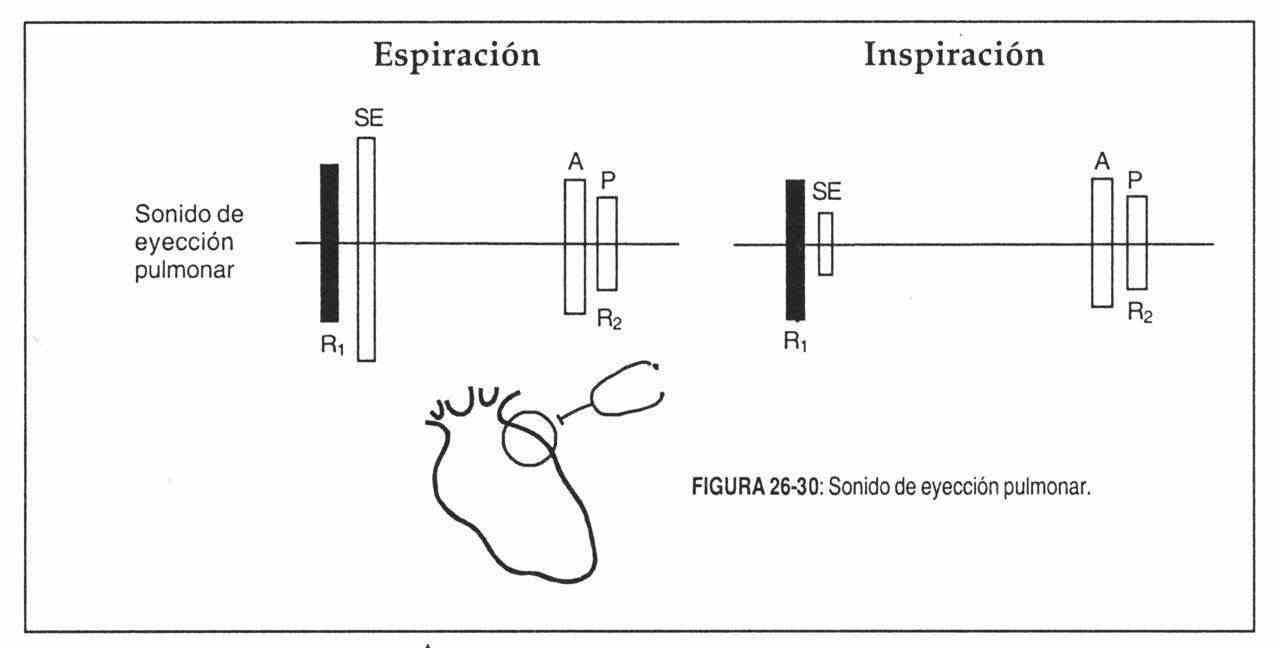
The pulmonary ejection sound is more easily identified. It is limited to the second or third left parastemal intercostal space, where the physiological splitting of the first sound is not perceived, and it varies enormously with breathing, being maximum on expiration (Figure 26-30). It is also accompanied by dilation of the pulmonary artery and is heard in pulmonary valve stenosis, pulmonary hypertension, idiopathic pulmonary dilation, ductus, and atrial septal defect. It is very rare in the latter condition. In the case of pulmonary stenosis, it is systematically absent in severe valve stenosis and in the infundibular form.
 2. Non-ejection clicks . Since the last century they were assigned an extracardiac etiology until JB Barlow, in 1963, proved cineangiographically that they corresponded to mitral clicks with or without valve regurgitation.
2. Non-ejection clicks . Since the last century they were assigned an extracardiac etiology until JB Barlow, in 1963, proved cineangiographically that they corresponded to mitral clicks with or without valve regurgitation.
Non-ejection clicks are the most sensitive and specific sign of mitral valve prolapse (MVP). They are related to the sudden tension of the redundant leaflets and the elongated chordae tendineae, coinciding with the maximum excursion of the prolapsed leaflets in the left atrium and not with the beginning of the prolapse.
On auscultatory terms, the mitral click sounds like a sharp, intense, brief vibration with a clicking sound, which is heard preferentially at the mitral and paramitral focus (Figure 26-31). Its most important characteristic is the variation in systolic location that it experiences with changes in position and with the passage of time. There are clicks that occupy the mesosystole in decubitus and that when standing up become, for example, end-systolic. An isolated mid-systolic click may, on further clinical examination, be replaced by one or more mid-systolic and end-systolic clicks.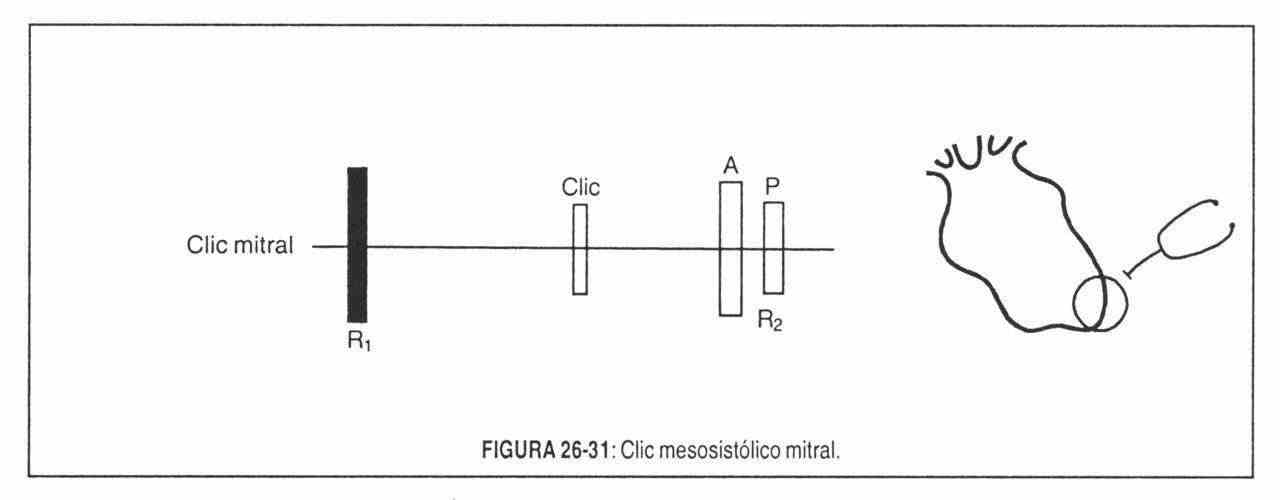
Amyl nitrite increases its intensity, contrary to what occurs in rheumatic chronic mitral regurgitation.
It should be noted that in mitral valve prolapse, early systolic clicks can be heard, which are sometimes difficult to distinguish from a first split sound.
It is important to bear in mind that Barlow and Cohén have auscultated non-ejection clicks in 16.5% of the normal population, and therefore it is difficult to affirm that this auscultatory finding always signifies pathology, since it may represent one end of the distribution curve normal. But if a mitral murmur is also auscultated, in this case the valve mechanism should always be considered abnormal.
On the other hand, non-expulsive click can also be found in other conditions: tricuspid valve prolapse, Ebstein's disease, and ventricular aneurysms.
Aerial noises
The presence of air, or air and fluid in the precordium, pleura, or mediastinum produces, with the heartbeat, various sounds that are generally systolic and heterogeneous.
In traumatic, surgical or spontaneous emphysema of the mediastinum (Hamman's disease), multiple noises, cracking or metallic, occur that resemble the sounds caused by crumpling a piece of cellophane or walking on dry snow. They are heard in the apex and the mesocardium, and their intensity is highly variable. Sometimes they are only heard in a single decubitus or in a single respiratory phase. They are known as a Hamman sign or crunch.
In the accumulation of air and liquid in the pericardium (heme or hydropneumopericardium), a splashing noise appears, similar to that produced by the wheel of a water mill, rhythmic by the heartbeat. Sometimes the patient himself perceives it. It can be auscultated after cardiac operations, by opening the pericardial sac, in hydropneumothorax, etc.
Pericardial packs
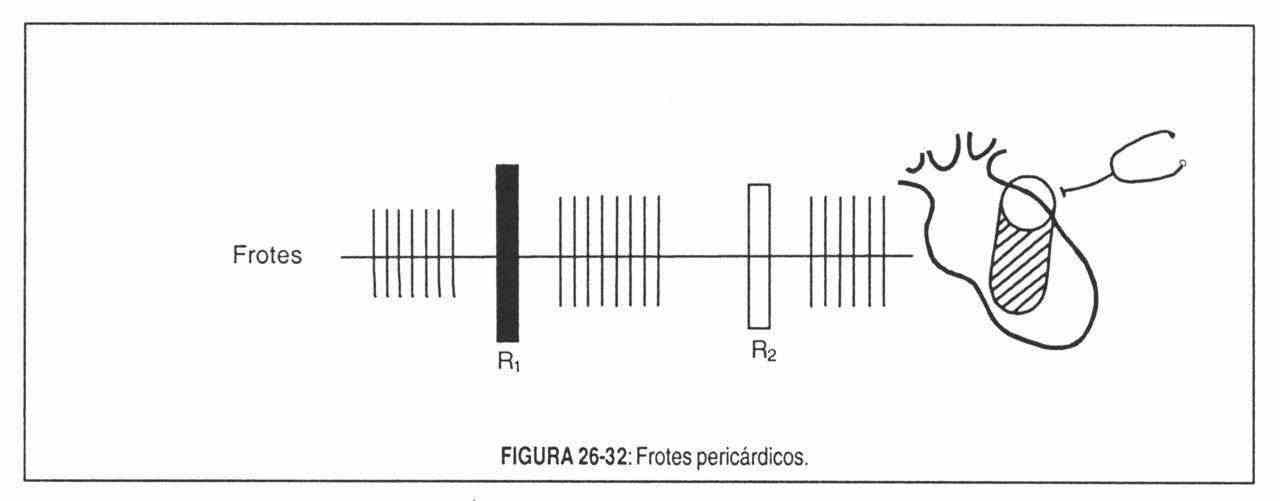 The de-pulping of the pericardial leaves generates, with cardiac action, a set of sounds known as pericardial rubs. Because of their heterogeneity, shortness, and high pitch, they are not considered murmurs but rather aggregated heart sounds (Figure 26-32).
The de-pulping of the pericardial leaves generates, with cardiac action, a set of sounds known as pericardial rubs. Because of their heterogeneity, shortness, and high pitch, they are not considered murmurs but rather aggregated heart sounds (Figure 26-32).
They are heard in both silences, but are more numerous in systole. They have a typical superficial character, of being heard so close to the ear that they immediately suggest pericarditis.
They are described as scraping, rough, harsh, similar to the "noise of falling: nine pe: other times they are very soft. Their intensity is also variable.
Its quality and breadth can be modified evolutionarily. It is located in any part of the precordium and it is characteristic that they vary with the position of the patient. They should be sought: both decubitus, dorsal and prone, in lateral decubitus and in an upright position, leaning the patient forward.
Pericardial rubs occur at three special moments in the cardiac cycle: 1 systolic, during rapid expulsion; 2) early diastolic, during rapid ventricular filling; 3) presystolic, during atrial systole. At these times of the cycle, the friction between the pericardial leaves is maximum.
Any process that generates roughness or discolouration on the pericardial surfaces will cause rubbing, which will disappear if a spill is installed that separates the leaves from the pericardium.
It is also possible to find variations with breathing in the case of pleuropericarditis.
Heart murmurs
Blood circulation throughout the cardiovascular system is silent because the current is laminar; In other words, the flow moves in the form of concentric sheets whose velocity is greater in the center of the current, due to friction.
When the blood flow loses its laminar character, it generates audible vibrations. The auditory perception of these vibrations is called a murmur.
Murmurs are actually heart sounds, but their duration is longer and their meaning is different. They are perceived with a blowing, harsh, low or high pitched tone, and occupy the silences, covering or not covering the noises.
Its character is not always impressive, as its name seems to indicate. That is why English-speaking authors call them "murmurs" (murmurs), a term that does not conjecture about the auditory character of vibrations.
There are several theories that try to explain the mechanism of production of murmurs. They will be analyzed briefly.
-
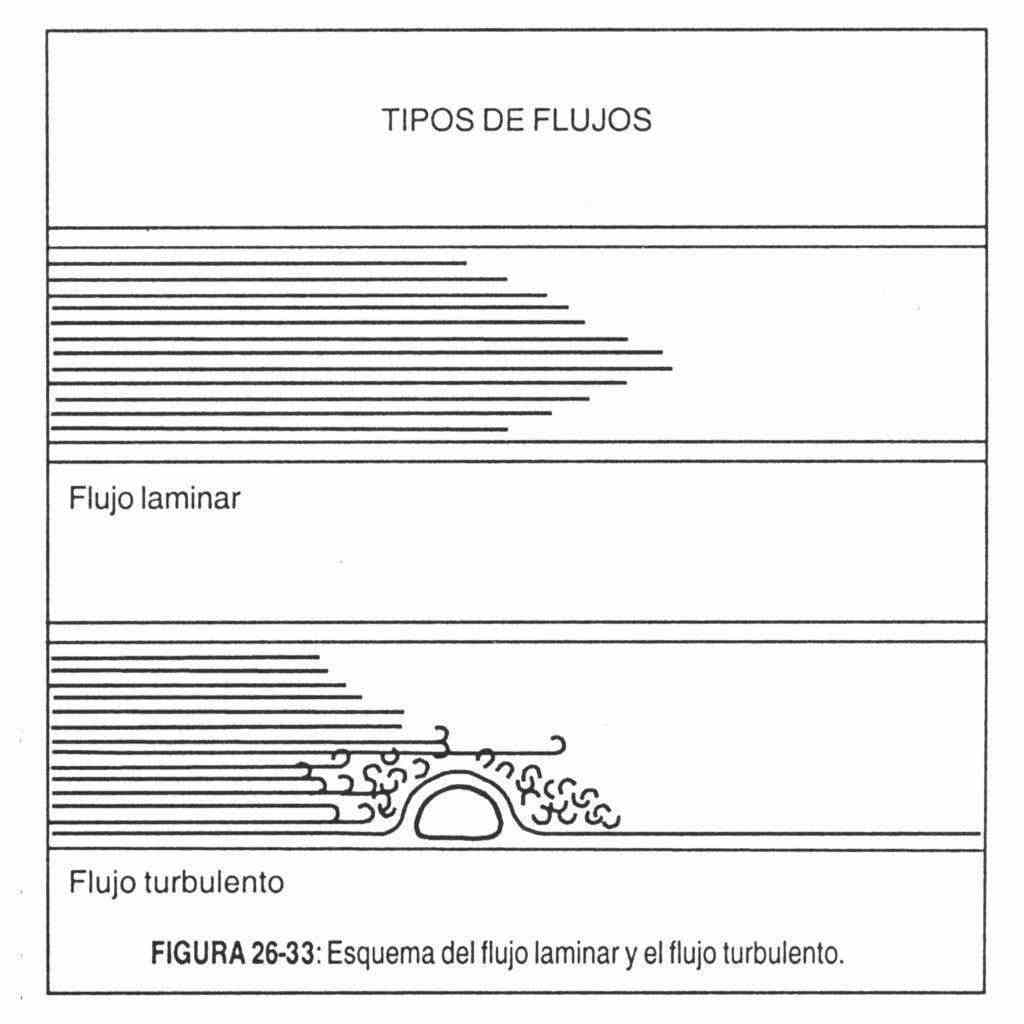 Turbulent flow theory . It is the most widely accepted (Figure 26-33). According to it, the puffs are the result of turbulence in the current flowing at high speed.
Turbulent flow theory . It is the most widely accepted (Figure 26-33). According to it, the puffs are the result of turbulence in the current flowing at high speed.
It is this turbulence that would generate audible vibrations (murmurs) and, if it is intense, palpable vibrations (thrills) that are perceived as a characteristic tremulous sensation. According to Leatham, there are three fundamental factors - not the only ones - that intervene in the production of turbulence and, therefore, of blows: 1) increase in the current through normal or abnormal valves; 2) forward flow through a stenosed, irregular valve, or into a dilated vessel (normal direction); 3) backward flow through insufficient valve or congenital defect (eg, ventricular septal defect) (reversed direction). These factors are often combined with each other.
The speed of a liquid at which laminar flow becomes turbulent is called the critical speed. When a local factor is added (valve stenosis, for example) turbulence occurs with lower speeds. But the fundamental fact to remember is the central importance of speed in the genesis and interpretation of murmurs. The speed of the current (turbulence factor) is the result of pressure differences between the heart and the great vessels and the heart chambers with each other. And this pressure difference (gradient) is responsible, not only for the breath, but for its shape, type and moment. - Theory of eddies or "fluctuating vortices" . According to her, the obstacle to a liquid stream would generate eddies or "fluctuating vortices", leaving a trail of them and generating acoustic energy.
- Theory of cavitation . It is based on the production of bubbles in a liquid due to the local pressure drop. This drop would occur when the flow is fast enough. The bubbles split and collapse, generating noises.
- Jet impact theory . According to her, the murmurs would originate from the high-speed impact of the blood stream on the cardiac or vascular structures, with their vibration. This is how certain fibrous thickenings of the parietal endocardium, the leaflets and the intima of the arteries are observed, produced by the impact of a high speed blood jet ("jet lesions"). This theory also explains the direction of the irradiation of the murmurs. Thus, in mitral regurgitation, when the jet hits the external wall of the left atrium, the murmur radiates towards the axilla; if, on the contrary, it reaches the inner wall, the murmur spreads towards the mesocardium.
- Flapping theory . According to her, the structures would vibrate due to pressure differences, as occurs in elastic tubes.
In conclusion, it can be said that, although the physical mechanisms that cause murmurs are still not well understood, apparently the most important factors in their production are: a) the increase in the speed of blood flow, whether due to passage through from a narrowing (stenosis) or from an insufficient valve, which causes fluctuating turbulence or eddies or an impact jet; b) the vibratory properties of the heart and vessels, of which little information is still available, and c) the decrease in viscosity, which acts only in certain conditions (anemia).
Causes of murmurs . On physical examination, murmurs are found in the following conditions:
- Valve narrowing (stenosis). The passage of blood must be made here from a chamber of higher pressure to another of lower pressure through a valve stenosis, causing a jet of greater or lesser speed, which causes an acoustic phenomenon, a murmur. Aortic and pulmonary strictures cause systolic murmurs; mitral and tricuspid stenosis, diastolic murmurs.
- Postvalvular dilatations. Even with normal valves, a murmur may appear if the cavity distal to the valve is dilated, which is why the latter behaves as "relatively" stenotic: aortic or pulmonary systolic murmurs due to dilation of the aorta or pulmonary; Diastolic murmurs (rumblings) mitral or tricuspid due to dilation of the left or right ventricle.
- Valvular regurgitation (insufficiency). In this situation, the murmur occurs when the blood flows with a certain speed in the opposite direction to normal, through an abnormal valve orifice, generating turbulence and impacts. This is the case of mitral and tricuspid regurgitation in systole and aortic and pulmonary regurgitation in diastole.
- Abnormal communications between heart chambers or between vessels. Here the genesis of the murmurs is in relation to the differences in resistance to flow and pressure between them. Interventricular and interatrial communications, patent ductus arteriosus, etc., are examples of this type.
- Increased flow rate. Due to the formation of eddies and / or turbulence, systolic murmurs are produced in hyperthyroidism, pregnancy, anemia, etc.
- Vascular narrowing. The narrowing of a vessel (aortic coarctation, for example) is capable of generating a murmur by a mechanism similar to that of valve stenosis.
- Vibrant solid structures. The presence of solid vibratile structures in the bloodstream (broken tendon cord, for example) can generate very intense murmurs, sometimes of musical timbre.
Qualities of the murmurs . In the study of murmurs, the following properties should be taken into account fundamentally:
-
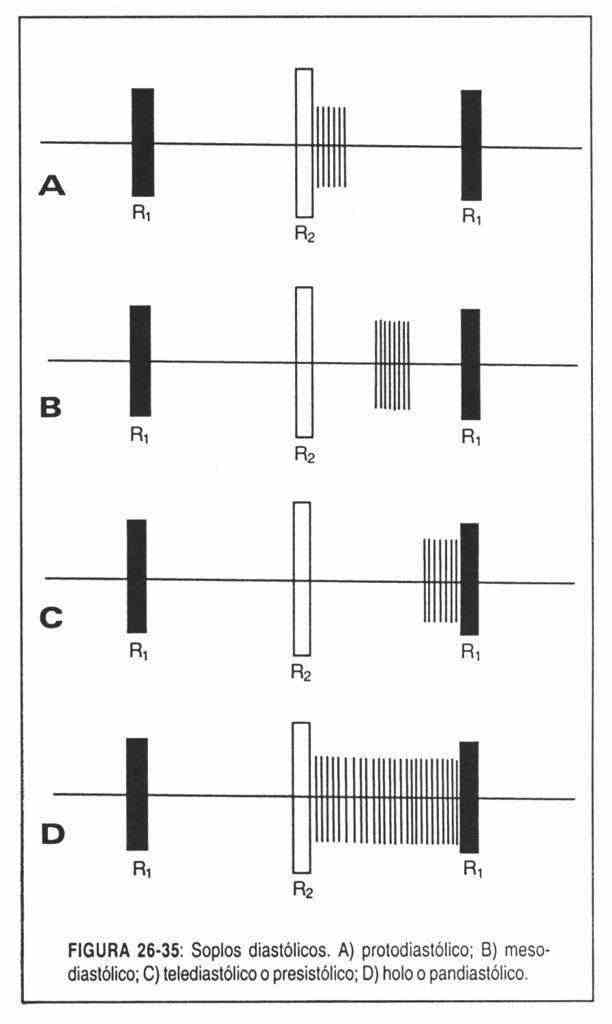
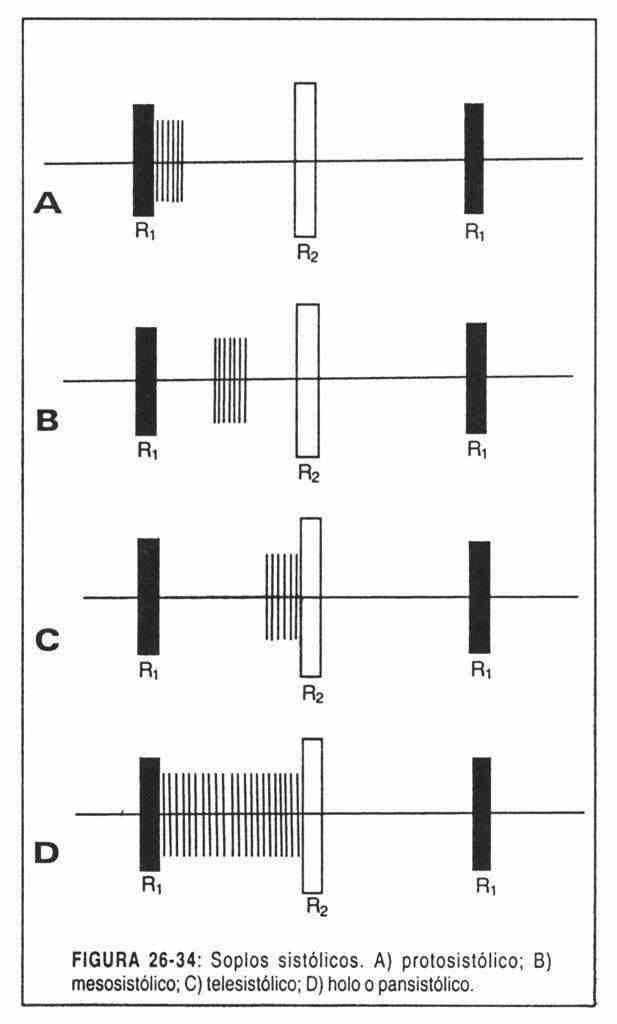
a) Systolic. They manifest between the first and second noises ("little silence"). These, in turn, can be (Figure 26-34):
- Holosystolic or pansystolic: they occupy the entire interval between the first and second noises.
- Protosystolic: if they are only in the first half of the systolic space.
- Mesosystolic: if they occupy the middle part.
-
Telesystolic: if they occur at the end B of systole.
If the murmur occupies more than half of the systole, it will be proto-mesosystolic or mid-systolic.
-
Diastolic. When the second and first noises are located between q. They can be (Figure 26-35):
- Holodiastolic: occupy the entire interval.
- Protodiastolic: when they follow the second noise, during the first part of diastole.
- Mesodiastolic: they begin a time after the second noise, from which they are separated by a silent interval and occupy the middle part of diastole.
- Presystolic or end-diastolic: when they occupy the end of diastole, preceding the first noise.
- Continuous. When they occupy both times of the cardiac cycle, without interruption and forming a single blowing phenomenon. The continuous murmur must be differentiated from the association of a systolic murmur with a diastolic murmur, or a "systolo-diastolic" murmur, which can occupy the entire cycle but shows a strangulation at the level of the second sound that clearly separates them.
The intensity of the murmurs . The intensity of the murmurs is a function of the amplitude of the vibrations, and is not proportional to the pathology or hemodynamic disturbance that originates it. There are intense murmurs due to inconsequential conditions, but which generate great turbulence.
 Conversely, a large, low-velocity flow through an ASD, for example, is completely silent, with no murmur at all.
Conversely, a large, low-velocity flow through an ASD, for example, is completely silent, with no murmur at all.
In addition, other factors that alter the intensity of a murmur must be taken into account (chest wall thickness, emphysema, etc.), of which the tidal volume must be mentioned: in aortic stenosis, for example, the loud murmur it becomes weak when left ventricular failure is installed.
In any case, the intensity of a murmur should always be determined, using some sort of classification. Levine and Harvey propose one in six grades:
Grade 1: very weak murmur, barely perceptible
Grade 2: slight, but easily audible
Grade 3: moderately intense
Grade 4: intense
Grade 5: very intense, with thrill
Grade 6: maximum intensity murmur, audible even when the stethoscope is separated from the chest wall. It has a thrill, that is, a palpable vibration.
The use of this scale presents some practical difficulties. The determination of grades 1,2 and 6 is usually not difficult, but the discrimination between grades 3,4 and 5 is usually too subjective. For this reason, some Services, such as the London and Mexico Institutes of Cardiology, use a simplified classification of six degrees:
Grade I: weak, not even audible in the first seconds of auscultation
Grade II: moderate, clearly audible
Grade III: strong, with thrill
Grade IV: very loud, audible before applying the stethoscope to the chest wall
In any case, and whatever the method used, the degree of intensity should be noted by putting the scale used in the denominator: for example, 2 / 4.3 / 6, etc.
Frequency, pitch or height of the murmurs . The pitch of the murmurs depends on the number of vibrations per second (cycles per second), and is thus divided into high (treble), medium and low frequency (bass) murmurs. High-pitched murmurs are better perceived with the membrane stethoscope, while the bell will facilitate auscultation of low-pitched murmurs.
High-pitched murmurs are primarily due to high current velocity, caused by a large pressure gap (gradient) through small orifices, as in aortic or pulmonary regurgitation murmurs.
Severe murmurs, on the other hand, are caused by a low-velocity current with small pressure drops and large communication holes, as is the case of the diastolic murmur of mitral stenosis.
In an intermediate position are the systolic murmurs. The speed is high, with large gradients, and they are best heard with the membrane stethoscope.
Timbre or quality of the murmurs . It is a subjective impression of the listener and is particularly interested in some murmurs. The timbre nuances the acoustic characteristics of a murmur and makes it often unmistakable.
In auscultatory practice, murmurs can be divided according to their tonal qualities or "timbre" into:
- Mild murmurs, which may be aspiration (such as the diastolic murmurs of aortic regurgitation) or expiratory.
- Jet murmurs, usually more intense and heard as the escape of pressurized steam ("steam jet" murmur from mitral regurgitation).
- Rough or harsh murmurs, with a high content of low vibrations and frequently associated with thrills. An example is the murmur of aortic stenosis.
- Musical murmurs, formed by regular vibrations that can be of very high frequency: piant murmurs, which are heard as the sound resulting from rubbing wet glass with a finger.
There are many examples in the literature of suggestive expressions related to the timbre of murmurs.
Length or duration . This acoustic characteristic is the most useful criterion in the semiological diagnosis of murmurs. The length or nature of a murmur follows a precise parallel with the hemodynamic disturbance that generates it. It was Leatham who first discovered the relationship of murmurs with unevenness or pressure gradients conditioned by the present hemodynamic disorder.
It is on the basis of these pathophysiological criteria that murmurs can be classified, following Leatham's concepts.
A. Systolic murmurs
They originate from the passage of a centrifugal current from both ventricles, during ventricular systole. In other words, they are caused by a flow that leaves the ventricles forwards or backwards. They are high frequency murmurs. They can be divided into two groups:
- Ejection or expulsive systolic murmurs, mesosystolic, caused by the passage of current forward through the aortic and pulmonary valves, or immediately below or above them.
- Pansystolic regurgitation murmurs, due to the passage of current backward from the ventricles or atria, due to insufficient atrioventricular valves, or from one ventricle to another due to a septal defect.
Both types of systolic murmurs are discussed below.
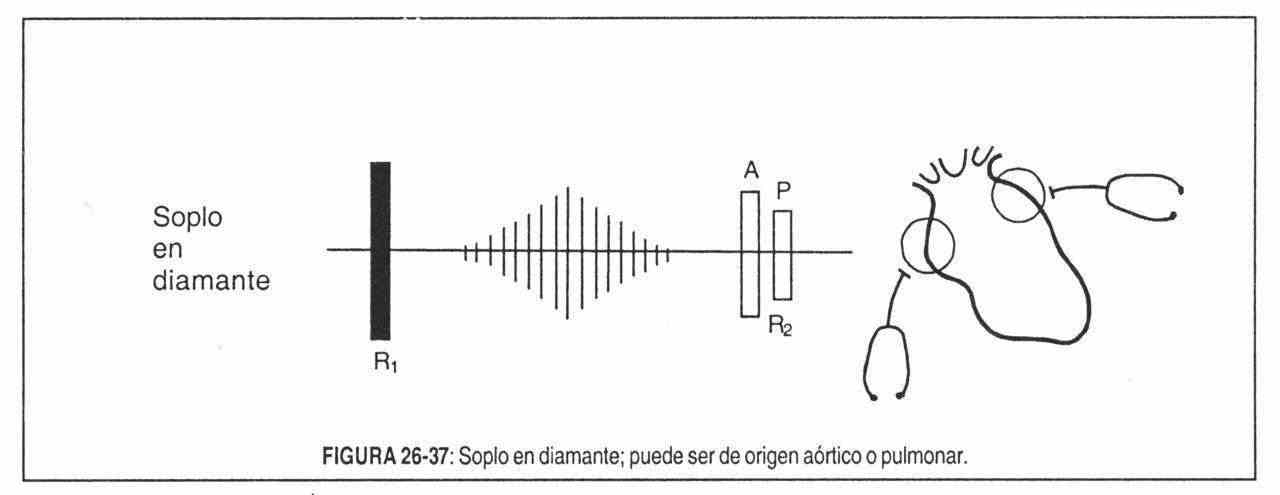 1. Mid-systolic ejection murmurs . Strictly speaking, they should be called expulsive systolic murmurs. They usually begin after a short interval separating them from the first noise, have their maximum amplitude at the time of maximum ventricular ejection velocity, and end before or next to the component of the second noise corresponding to the affected side. Thus, they have an increasing-decreasing or “rhomboid” or “diamond” morphology (Figure 26-37), since they are directly related to the transvalvular flow and therefore to the pressure gradient. They will be of low intensity at the beginning, they will increase in the maximum ejection phase and will decrease in the reduced ejection phase. This fact is fundamental.
1. Mid-systolic ejection murmurs . Strictly speaking, they should be called expulsive systolic murmurs. They usually begin after a short interval separating them from the first noise, have their maximum amplitude at the time of maximum ventricular ejection velocity, and end before or next to the component of the second noise corresponding to the affected side. Thus, they have an increasing-decreasing or “rhomboid” or “diamond” morphology (Figure 26-37), since they are directly related to the transvalvular flow and therefore to the pressure gradient. They will be of low intensity at the beginning, they will increase in the maximum ejection phase and will decrease in the reduced ejection phase. This fact is fundamental.
Models of this type of murmur are those of aortic and pulmonary stenosis.
Aortic valve stenosis . It is heard in the aortic area or focus, clearly irradiated on one side towards the neck vessels, especially on the right side, and on the other towards the mesocardium and apex. Cervical irradiation is such a frequent phenomenon that its absence should cast doubt on the diagnosis.
If the stenosis is mild or if the valve is not calcified, the beginning of the murmur is marked by the presence of an ejection click.
The murmur of aortic stenosis is generally loud, with a harsh timbre, almost always accompanied by a thrill, and is reinforced late (at 30-40 seconds) with the inhalation of amyl nitrite.
Since aortic valve closure normally precedes the pulmonary one, the murmur ends before the first component of the second sound. But when the mechanical systole of the left ventricle is prolonged, as it occurs in severe aortic stenosis, the valve closure is reversed (reversed splitting of the second sound) and the murmur can bypass the pulmonary component but always ends before aortic closure.
It is important to remember that the maximum amplitude of the murmur coincides with the apex of the pressure gradient between the left ventricle and the aorta. This vertex (and therefore the murmur) occurs the later the greater the gradient, that is, the more severe the valve obstruction. The murmurs of very severe stenoses thus have their maximum amplitude in the second half of systole.
Either way, whether the murmur is weak or short, an aortic stenosis will never be diagnosed in the absence of other signs of the condition.
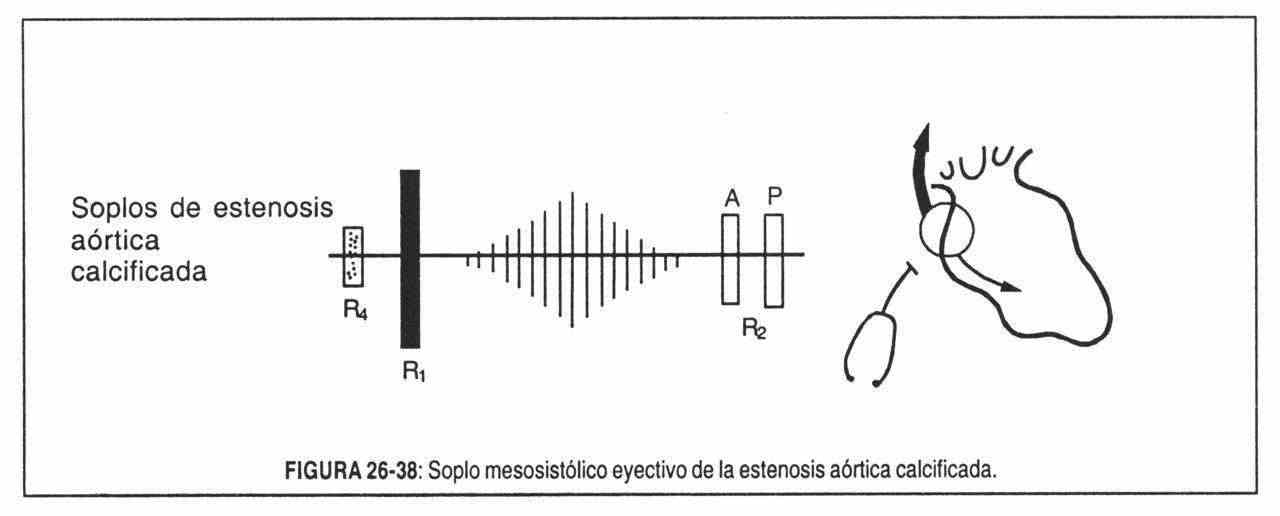 In older people, in the second right intercostal space, with clear radiation to the right side of the neck and at the point of maximum intensity, the systolic murmur of calcific aortic stenosis can be heard (Figure 26-38).
In older people, in the second right intercostal space, with clear radiation to the right side of the neck and at the point of maximum intensity, the systolic murmur of calcific aortic stenosis can be heard (Figure 26-38).
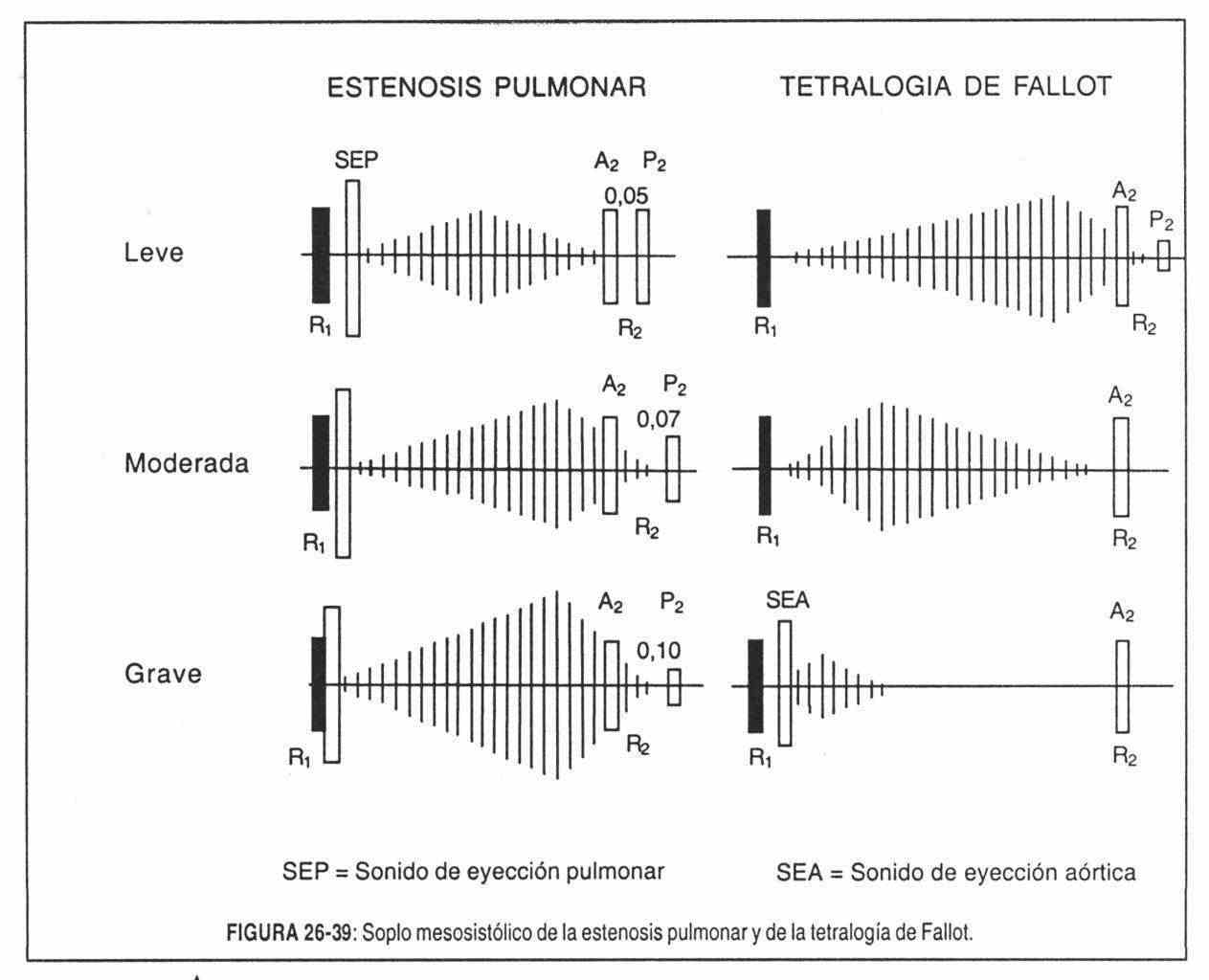 Pulmonary valve stenosis . These murmurs are also rhomboid or crescendo-decreasing, with mid-systolic accentuation, and invariably end before the pulmonary component of the second sound (Figure 26–39); they are heard in the pulmonary focus and in the left third intercostal space and do not radiate to the apex, unless the tip is formed by the right ventricle.
Pulmonary valve stenosis . These murmurs are also rhomboid or crescendo-decreasing, with mid-systolic accentuation, and invariably end before the pulmonary component of the second sound (Figure 26–39); they are heard in the pulmonary focus and in the left third intercostal space and do not radiate to the apex, unless the tip is formed by the right ventricle.
The intensity of the murmur ranges from 2/6 to 5/6 and, in general, the tighter the stenosis, the more intense the murmur tends to be, unless tricuspid regurgitation is installed or the right ventricle fails.
The time of maximum intensity of the murmur is, as in aortic stenosis, the later the tighter the stenosis. Thus, in severe cases it masks the aortic component of the second sound, but always ends before the first component, even though it is weak and very late.
The murmur is almost always accompanied by a thrill in the pulmonary area, and when the stenosis is not severe, a pulmonary ejection sound is heard. It should be remembered that in this pathology there is an audible expiratory doubling of the second noise. If the pulmonary stenosis is infundibular, as seen in tetralogy of Fallot (Figure 26-39) and other conditions, no ejection sound will be heard (it is in non-severe valve stenosis). Pulmonary valve closure noise is not heard in severe tetralogies of Fallot.
The behavior of the murmur of pulmonary stenosis with the Valsalva maneuver is typically a "right behavior": it decreases in intensity during the compression phase, but appears with maximum intensity from the first beats that follow the end of compression. Amyl nitrite intensifies it, by increasing venous return.
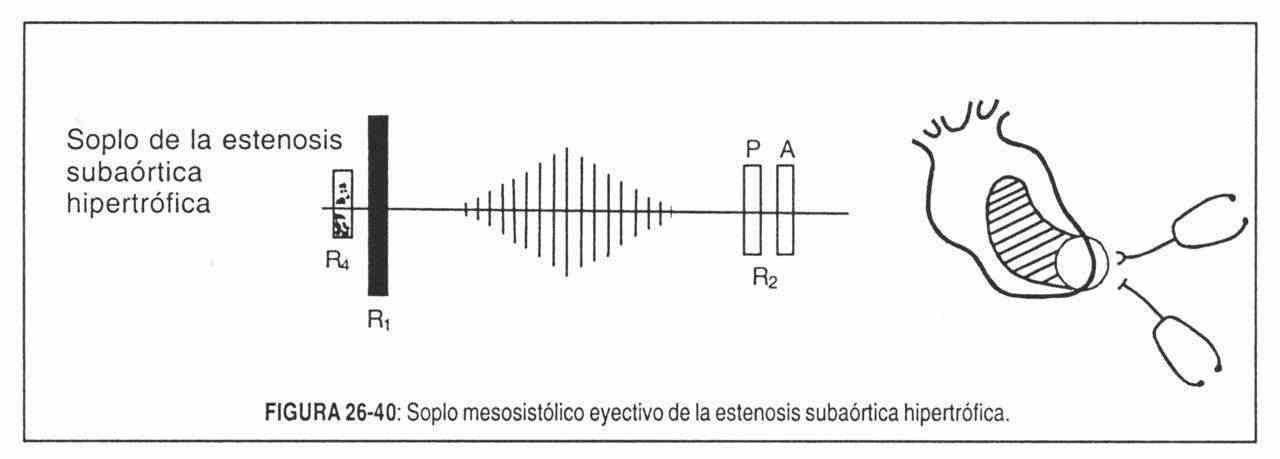 Subaortic stenosis . Aortic subvalvular stenosis differs auscultatory from valve stenosis by the absence of the proto-systolic click, at the epicenter of the murmur (mesocardium and / or apex) and by its radiation, which does not spread to the neck. It may intensify during the "pressure" phase of the Valsalva maneuver, while the administration of cardiac stimulant drugs (digitalis, isoproterenol) intensifies the murmur, in contrast to what occurs with valvular aortic stenosis (Figure 26-40). This murmur decreases with the squatting position, and increases when standing up and after extrasystoles. On the other hand, the characteristics of the carotid pulse are very different in both pathologies.
Subaortic stenosis . Aortic subvalvular stenosis differs auscultatory from valve stenosis by the absence of the proto-systolic click, at the epicenter of the murmur (mesocardium and / or apex) and by its radiation, which does not spread to the neck. It may intensify during the "pressure" phase of the Valsalva maneuver, while the administration of cardiac stimulant drugs (digitalis, isoproterenol) intensifies the murmur, in contrast to what occurs with valvular aortic stenosis (Figure 26-40). This murmur decreases with the squatting position, and increases when standing up and after extrasystoles. On the other hand, the characteristics of the carotid pulse are very different in both pathologies.
2. Regurgitation systolic murmurs
Regurgitant systolic murmurs are due to retrograde flow from the ventricles to the atria, or from one ventricle to another due to a septal defect. Generally, the murmur begins with the first sound and extends through the entire systole, sometimes covering the second sound. Valvular regurgitations and interventricular communications are then manifested by holosystolic murmurs in most cases, although there are certain conditions in which this length characteristic is not fulfilled.
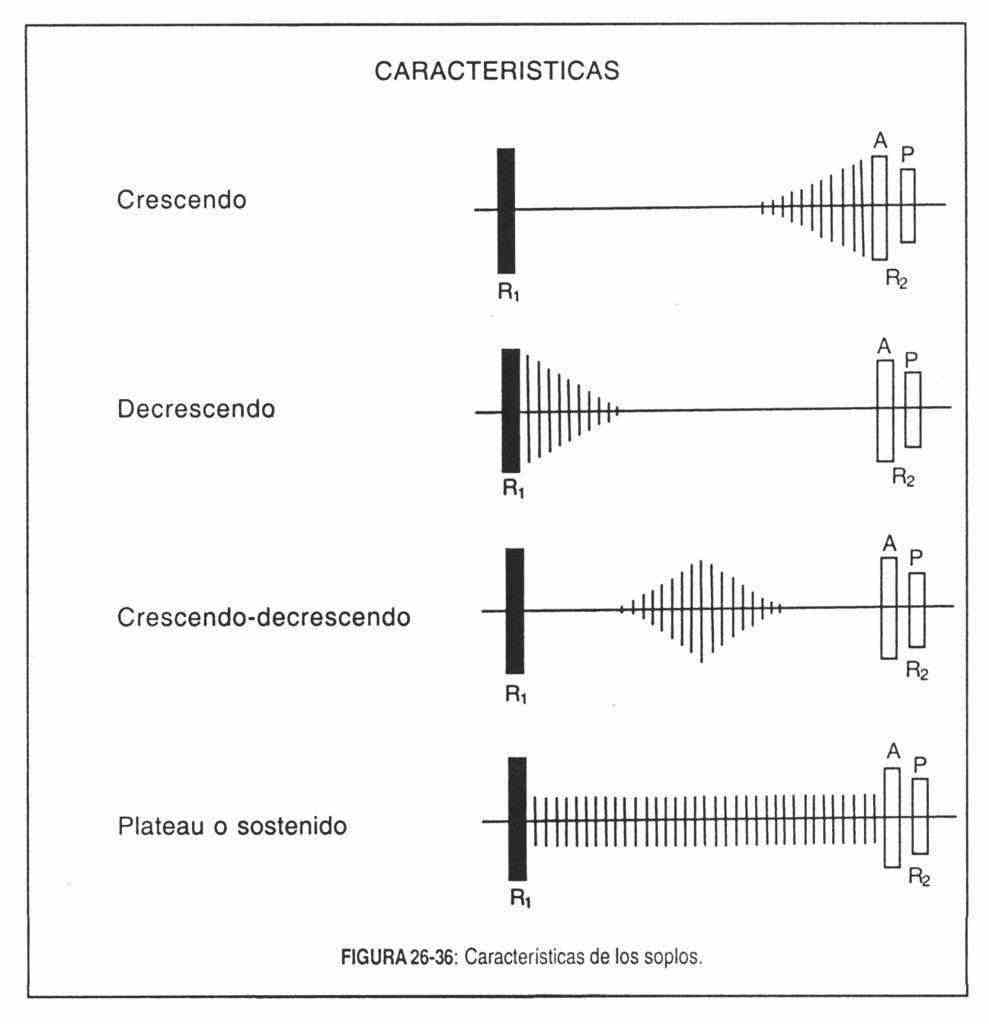
These regurgitant murmurs are long because the pressure drop that causes them is maintained throughout the entire systole. They are generated by the passage of current from a cardiac chamber that has higher pressure throughout systole than the chamber that receives it. Its intensity will then be uniform ("plateau") and there will be no interval between the heart sounds and the murmur, which extends between R1 and R2, and which ends up masking the latter.
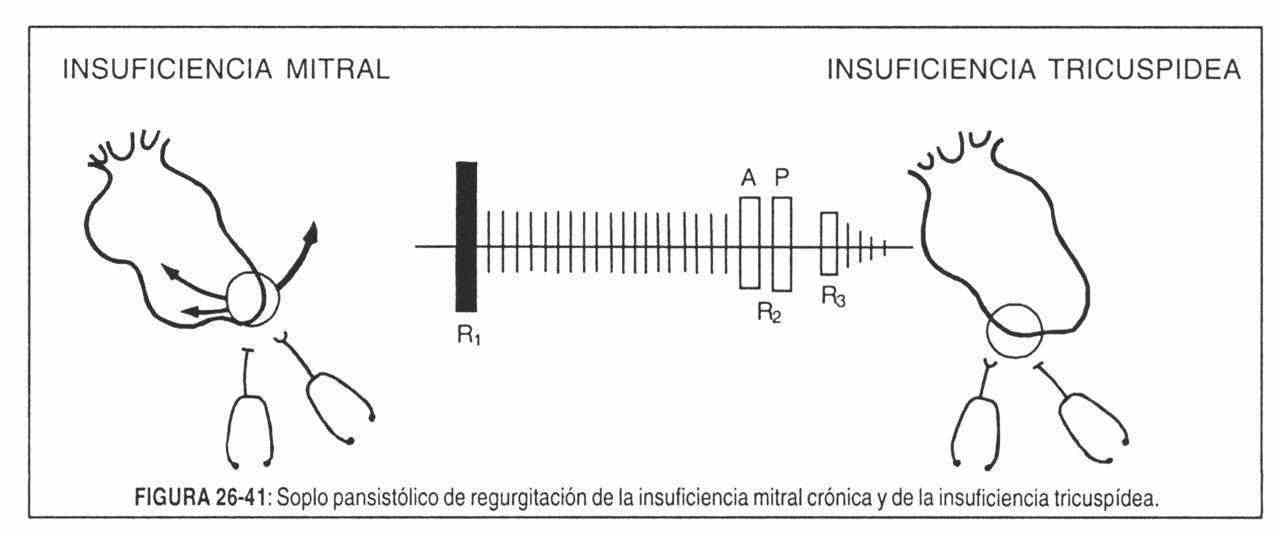 Pansystolic murmur of chronic mitral regurgitation . It begins immediately after the first sound, includes the second sound, is loud, long, sometimes accompanied by a thrill, maximum in the territory of the tip and radiates towards the armpit and the left scapula. It decreases markedly in intensity to the right of the sternum (Figure 26-41).
Pansystolic murmur of chronic mitral regurgitation . It begins immediately after the first sound, includes the second sound, is loud, long, sometimes accompanied by a thrill, maximum in the territory of the tip and radiates towards the armpit and the left scapula. It decreases markedly in intensity to the right of the sternum (Figure 26-41).
The murmur will decrease in intensity if atrial fibrillation is installed or in the presence of peripheral vasodilation due to heat or drugs (amyl nitrite) that facilitate the flow of current towards the aorta, reducing the regurgitant volume.
Although its most frequent morphology is the "plateau" variety, it may be decreasing due to a progressive increase in left atrial pressure.
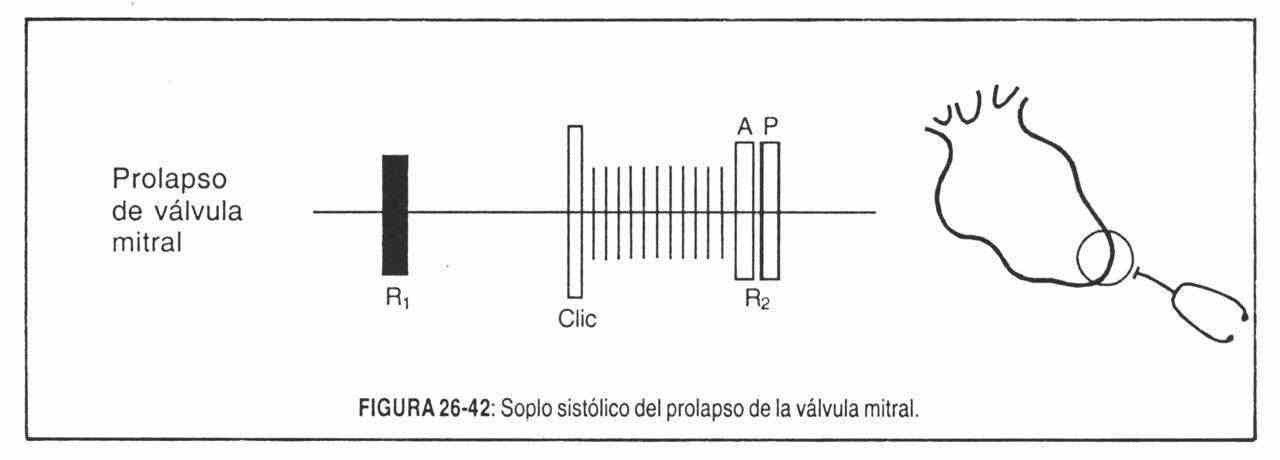 Systolic murmur of mitral valve prolapse . In this condition, the mitral valve has degenerative structural alterations that lead to eversion of the mitral blades in the left atrium during late systole, which can lead to mitral regurgitation (Figure 26-42).
Systolic murmur of mitral valve prolapse . In this condition, the mitral valve has degenerative structural alterations that lead to eversion of the mitral blades in the left atrium during late systole, which can lead to mitral regurgitation (Figure 26-42).
The most sensitive and specific sign of mitral prolapse are non-ejection, meso and end-systolic, mobile clicks. Another valuable auscultatory data is the end-systolic murmur, which is sometimes very high-pitched, musical, for which it has been compared to the goose squawk and called the end-systolic "honk" or "whoop". Sometimes it is not perceived at rest but after exertion. Its focus of maximum auscultation is the mitral area, and it is exaggerated with amyl nitrite or isoproterenol, contrary to what occurs in classic mitral regurgitation.
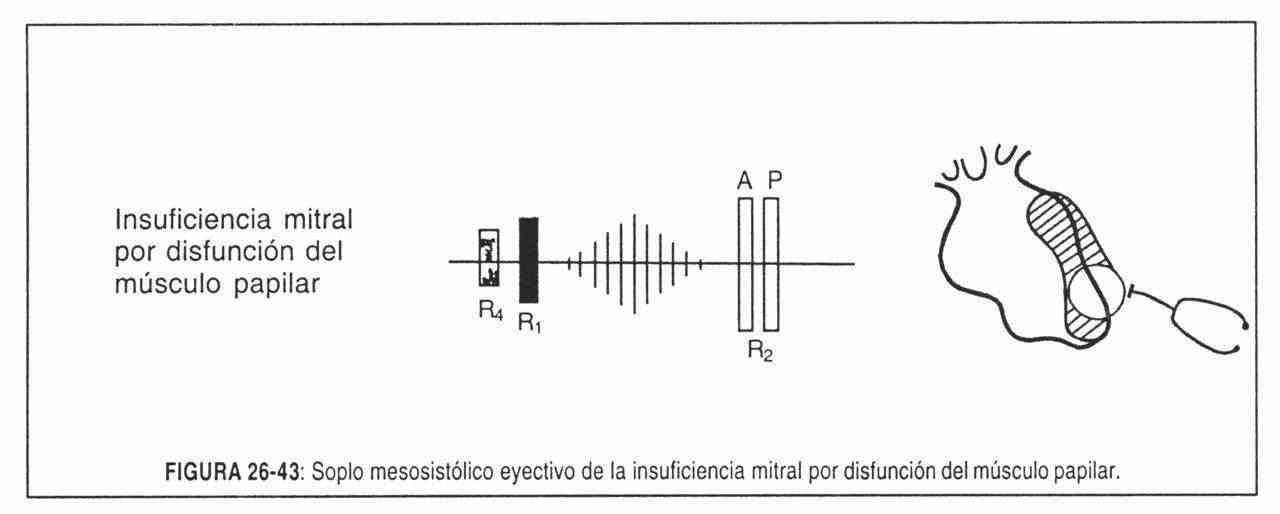 Systolic murmur of acute mitral regurgitation . In acute mitral regurgitation of any origin, the reflux of blood into a normal, tonic, and non-dilated left atrium, raises the pressure in the same and decreases the reflux as the systolic pressure in the left ventricle increases. The murmur, therefore, is decreasing and ends before the second noise. The first mitral sound is always present, although sometimes less intense, while the second sound can be unfolded by lengthening of the right ventricular systole. There may be a third sound from left ventricular failure and a fourth left sound (Figure 26-43).
Systolic murmur of acute mitral regurgitation . In acute mitral regurgitation of any origin, the reflux of blood into a normal, tonic, and non-dilated left atrium, raises the pressure in the same and decreases the reflux as the systolic pressure in the left ventricle increases. The murmur, therefore, is decreasing and ends before the second noise. The first mitral sound is always present, although sometimes less intense, while the second sound can be unfolded by lengthening of the right ventricular systole. There may be a third sound from left ventricular failure and a fourth left sound (Figure 26-43).
Systolic murmur of tricuspid regurgitation . As in chronic mitral regurgitation, the murmur of tricuspid regurgitation is pansystolic (Figure 26-41); it is heard with maximum intensity in the tricuspid area, or over the xiphoid appendix, but can be very intense at the tip if the right ventricle is very dilated. Thrill is less common than in mitral regurgitation. But the most important sign of the tricuspid pansystolic murmur - like all tricuspid murmurs - is its inspiratory reinforcement or Rivero-Carballo sign, caused by increased filling of the right cavities, due to increased venous return, as intrathoracic pressure decreases. .
To practice the Rivero-Carballo maneuver, the patient is instructed to inhale more deeply when the doctor raises his hand and to exhale when he lowers it. Sometimes you have to resort to the "repeated maneuver" that the Mexican author described: the patient is ordered to take several consecutive deep breaths, and then breathe in as deeply as possible.
Occasionally, along with the murmur, a third sound, and a mid-diastolic murmur, of tricuspid filling can be heard. Venous pressure will be elevated, and a right ventricular beat will be found.
Pansystolic murmur of ventricular septal defect . When the VSD proceeds without an increase in pulmonary pressure, the left ventricular pressure is much higher than the right throughout systole, and both left and right current and murmur are pansystolic. The murmur will then be identical to that of chronic mitral regurgitation: holosystolic, encompassing the first and second sounds, intense, almost always with thrills, with maximum intensity in the third or fourth left intercostal space, parastemal, horizontally irradiated.
With the Valsalva maneuver it is reinforced slowly, in a progressive and delayed way (from the sixth to the tenth beats that follow the test). In other words, it is a breath of "left behavior." The second sound has a wide but not fixed doubling, due to shortening of the left ventricular systole.
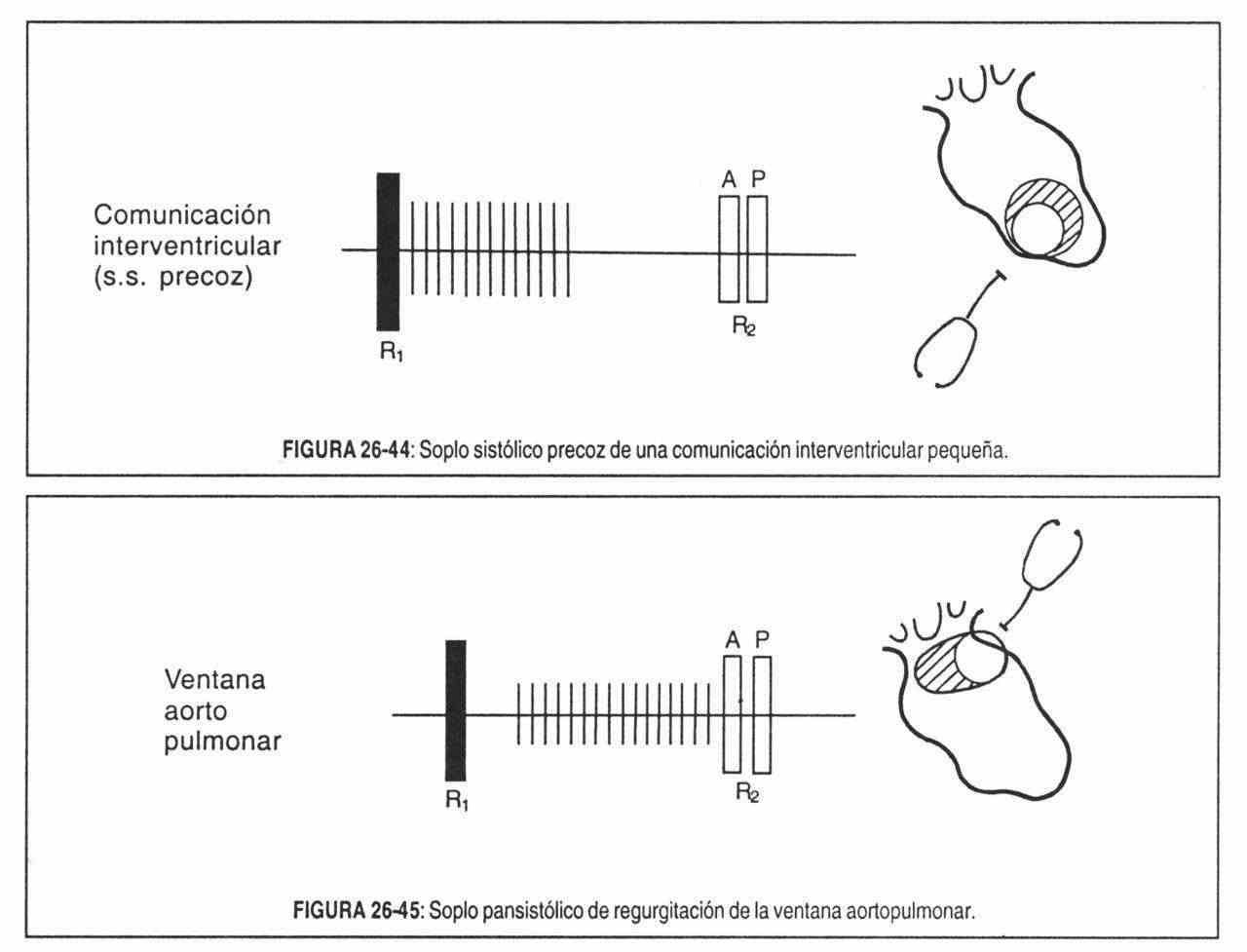 As progressive degrees of pulmonary hypertension develop, the murmur is no longer pansystolic, as the current decreases, and will subsequently become protomesosystolic (Figure 26-44), protosystolic, and then disappear as the central shunt is reversed. It is the stadium called "Eisenmenger complex".
As progressive degrees of pulmonary hypertension develop, the murmur is no longer pansystolic, as the current decreases, and will subsequently become protomesosystolic (Figure 26-44), protosystolic, and then disappear as the central shunt is reversed. It is the stadium called "Eisenmenger complex".
Holosystolic murmur from the aortopulmonary window . This pansystolic murmur does not start immediately after the first noise because the blood flow generates turbulence only when the aortic valve opens (Figure 26–45); progressive pulmonary hypertension makes the diastolic portion of the murmur disappear.
B. Diastolic murmurs
These murmurs are caused by the passage of centripetal current to both ventricles during ventricular diastole. Of course, they occur in the great silence, that is, between the second and the first noise.
In clinical practice, it is good judgment to consider all diastolic murmurs as "pathological", since non-pathological murmurs are exceptional. In contrast, non-pathological systolic murmurs are not uncommon, as will be seen later. Due to their production mechanism, they are classified into two groups:
1. Early diastolic regurgitation murmurs, due to the passage of blood flow backwards (reversed direction), through the sigmoid valves (insufficient aortic and pulmonary) or a persistent ductus (diastolic element of the continuous murmur).
2. Ventricular filling murmurs, by the passage of current forward (normal direction), from the atria to the ventricles through the atrioventricular valves. They can be: a) rapid filling phase: mid-diastolic ("rumbling"), and b) atrial systole phase: presystolic.
1. Regurgitation diastolic murmurs . They begin immediately after insufficient sigmoid valve closure, due to the rapid installation of a large pressure gap between the great vessels and their respective ventricles. Then they decrease progressively (decreasing murmurs) as diastolic blood pressure falls.
As the differences or unevenness of pressure are extreme (more than 100 mm Hg), the blood flows at high speed, for which the murmurs are of high frequency; strictly speaking, they are the highest frequency heart murmurs. The membrane stethoscope or the bare ear and, above all, maximum attention in auscultation of early diastole, are the best methods to detect them.
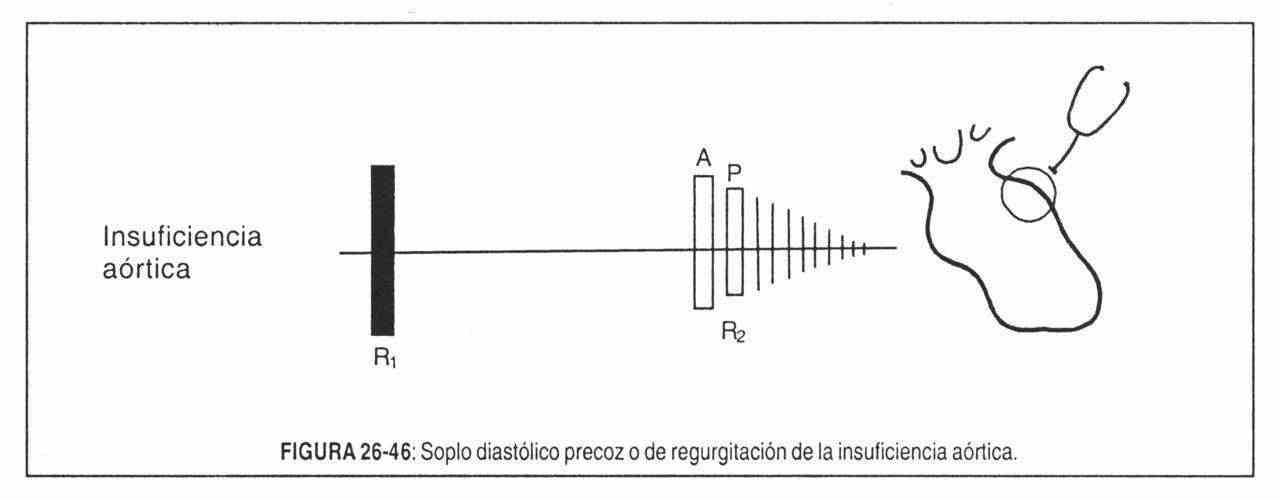 Early diastolic murmur of aortic regurgitation (Figure 26-46). It begins on the aortic component of the second sound, is decreasing and is auscultated with an epicenter in the aortic focus or the left sternum (left second-third intercostal space) and radiates along the sternal border towards the tricuspid and mitral foci. It is high-frequency, smooth, with an "aspiration" timbre, and is best perceived in a sitting position, with the patient reclined forward, and in post-respiratory apnea (Figure 26-47).
Early diastolic murmur of aortic regurgitation (Figure 26-46). It begins on the aortic component of the second sound, is decreasing and is auscultated with an epicenter in the aortic focus or the left sternum (left second-third intercostal space) and radiates along the sternal border towards the tricuspid and mitral foci. It is high-frequency, smooth, with an "aspiration" timbre, and is best perceived in a sitting position, with the patient reclined forward, and in post-respiratory apnea (Figure 26-47).
If the diastolic murmur is short, it will be a mild regurgitation, but if it is long, occupying more than half of diastole, the defect will be severe.
The most common cause of aortic regurgitation is rheumatic insufficiency or Corrigan's disease, which is very often accompanied by some degree of aortic valve stenosis and mitral injury.
The timbre of the murmur may be musical, "dove coo" or "seagull cry" type in sigmoid rupture or eversion, or in valve perforations. This is the case of syphilitic etiologies or infective endocarditis.
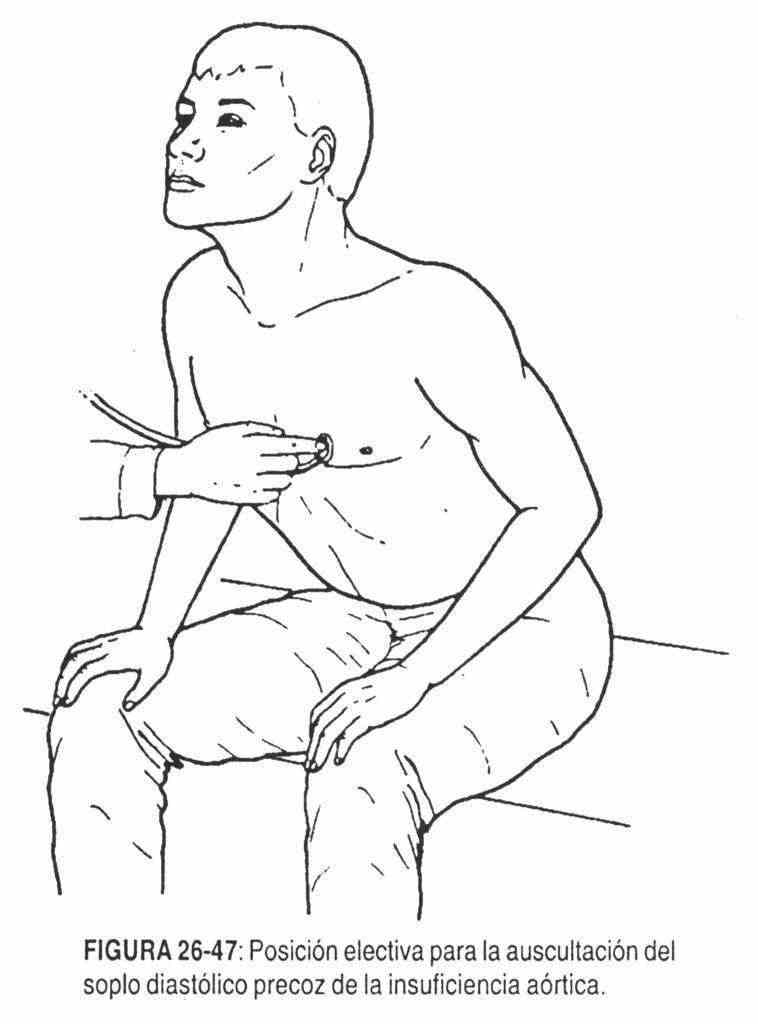 With the Valsalva maneuver, the murmur slowly and progressively intensifies in the post-pressure ("left") phase, while amyl nitrite quenches it. Increased peripheral resistance (with phenylephrine) increases regurgitation and murmur.
With the Valsalva maneuver, the murmur slowly and progressively intensifies in the post-pressure ("left") phase, while amyl nitrite quenches it. Increased peripheral resistance (with phenylephrine) increases regurgitation and murmur.
There will also be a wide and abrupt pulse (celer), a left ventricular beat displaced to the left and downwards and, frequently, an associated aortic expulsive murmur, giving the cadence of swaying.
A weak, low-frequency diastolic filling murmur with presystolic reinforcement is sometimes heard at the apex, produced by the impingement of the regurgitant jet against the anterior mitral leaflet during diastole. This is the functional Austin Flint murmur, which disappears with amyl nitrite, the reverse of the diastolic murmur in mitral stenosis.
Early diastolic murmur of pulmonary failure . It is similar in shape and time to the aortic, early decreasing diastolic, high frequency, but limited to the second and third left intercostal spaces and with a loud second noise.
It is almost always a functional murmur due to pulmonary valve regurgitation secondary to pulmonary arterial hypertension. It is called the "Graham Steell murmur" after the man who described it in 1888 (Figure 26-48).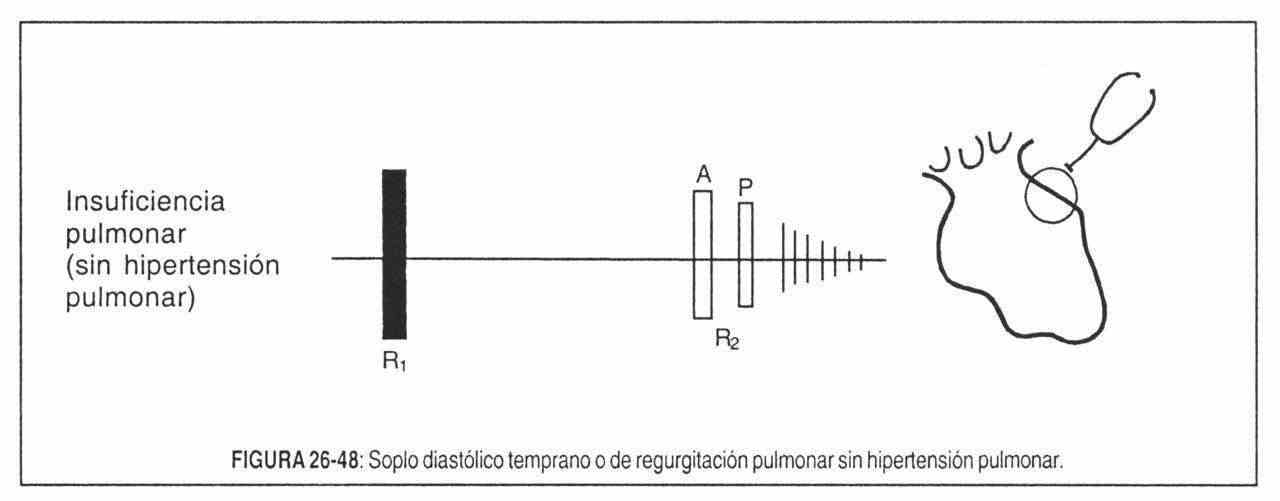
The differentiation between aortic and pulmonary regurgitation should not be based only on the characteristics of the murmur but, and fundamentally, on the analysis of the other signs, especially the presence or absence of pulmonary arterial hypertension, an almost obligatory companion of the Graham Steell murmur.
2. Diastolic filling murmurs . Ventricular filling occurs in two instances: the rapid, passive filling phase, caused by the atrioventricular pressure drop, and the active filling phase created by atrial contraction or systole, which completes ventricular filling.
When the atrioventricular gradient is high enough for current to flow through the mitral or tricuspid valve at a relatively high speed, turbulence and therefore murmurs occur at the time of maximum flow: rapid filling period (mid-diastolic) or atrial systole period (presystolic). If the gradient is large (greater than 10 mmHg) and is maintained throughout diastole, the murmur will occupy the entire diastole, simulating a pandiastolic murmur, but always beginning appreciably after the second noise.
Compared to diastolic regurgitation murmurs, mitral filling murmurs are of low frequency, since the pressure gradients are relatively small, so the blood cannot take great speed. Thus, they are best perceived with the bell stethoscope gently supported.
3. Mid-diastolic murmurs . Mid-diastolic murmurs always begin after the diseased atrioventricular valve has opened, so they are almost always separated from the second sound by a more or less long silent interval, which serves to differentiate them from regurgitation murmurs
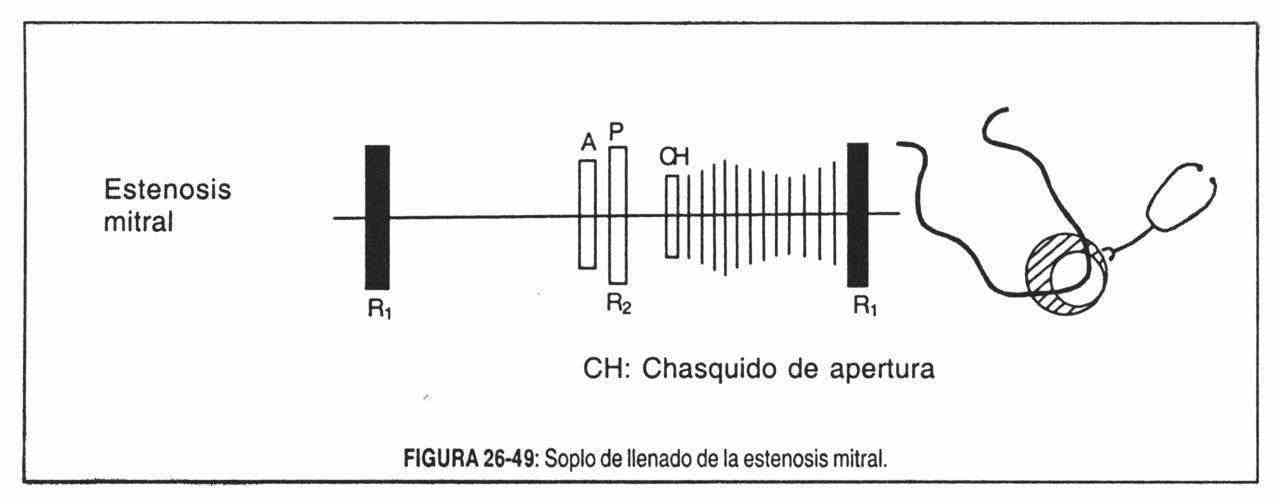 Mid-diastolic murmur (rumble) of mitral stenosis (also called "rolling," "dragging," or "roulement") (Figure 26-49). It is located in or near the apex region; it is a low-frequency, prolonged, harsh, dull and rumbling, which
Mid-diastolic murmur (rumble) of mitral stenosis (also called "rolling," "dragging," or "roulement") (Figure 26-49). It is located in or near the apex region; it is a low-frequency, prolonged, harsh, dull and rumbling, which
Laennec compared it to the sound of "sawing wood." It is frequently associated with a diastolic thrill, is usually preceded by an opening click, and is followed by a presystolic murmur, giving the impression of a harsh murmur with a presystolic reinforcement and an abrupt ending (first intense and clicking noise).
This is what Duroziez described as mitral stenosis onomatopoeia: rrou-ffut-tatá.
In this rhythm, "rrou" would be the diastolic rumble; "Ffut", the presystolic murmur, with the final t representing the first reinforced sound; and tata would be constituted by the second noise and the mitral opening click.
It should be noted that in general, the precordial area where it can be auscultated is relatively small and its irradiations are scarce. When it is not very intense, the left lateral decubitus should be used.
In mitral stenosis with atrial fibrillation, the presystolic murmur will disappear when the atrial contraction is suppressed, in which case the rumbling fades progressively before the first sound.
Functional mitral rumblings. These rumbles are from "relative mitral stenosis" and are produced by the following factors: a) increased transmission flow or "hypervolemic rumblings"; b) dilation of the left ventricle, which makes the normal mitral valve relatively narrow for a large ventricle.
Within the hypervolemic rumblings, the one caused by the active mitral valvulitis of acute rheumatic carditis is observed, where some alteration of the valve structure causes turbulence and gives rise to a weak murmur (Carey Coombs murmur), short, low frequency, It is magnified if there is fever or anemia, and it has an increasing-decreasing character. Other hypervolemic rumblings are sometimes seen in severe mitral regurgitation, ductus, VSD, severe anemias, and hyperthyroidism.
Due to left ventricular dilation rumbling can be heard in those conditions that severely dilate the left ventricle (aortic stenosis, cardiomyopathies, heart failure of any origin).
Also a left atrial myxoma can give rise to a mitral rumbling by interfering with the normal opening of the valve; its most important characteristic is the variation during auscultation, with changes in position.
Diastolic murmur of tricuspid stenosis . Like mitral stenosis, this pathology produces a mid-diastolic rumbling, but with a much higher frequency, and of maximum auscultation in the tricuspid focus and, like all tricuspid signs, characteristically increases on inspiration (Rivero-Carvallo sign). In atrial fibrillation it acquires a characteristic crescendo-descrescendo shape.
There are also tricuspid rumbling (wide atrial septal defect, severe tricuspid regurgitation), due to right ventricular dilation or interference with the valve opening (right atrial myxoma).
Presystolic murmurs . They are linked to the pressure drop created by active atrial contraction, when atrial systole completes ventricular diastolic filling.
As they are actually "expulsive" murmurs from the atrium to the ventricle through a narrow valve orifice, their actual morphology is rhomboid, increasing-decreasing.
This is seen when the atrio-ventricular conduction time is prolonged (PR interval greater than 0.20 sec). But they usually have a crescent appearance and end abruptly with the first noise, usually reinforced.
When atrial contraction is absent, as in atrial fibrillation, a presystolic murmur does not occur.
The presystolic murmur of mitral stenosis is harsh, of low frequency, generally increasing this is particularly evident when it accentuates a rumbling that is fading and ends suddenly in a first loud noise. The presystolic murmur and the first loud noise are the true harbingers of mitral stenosis, since they are generally the first signs of this condition.
The presystolic murmur of tricuspid stenosis is higher pitched and more rhomboid in morphology than the mitral murmur. On the other hand, it is frankly reinforced by inspiration.
C. Continuous rooms
Continuous murmurs are those that occupy systole and diastole, producing the impression of a single murmur on auscultation. They are due to the continuous passage of current from a region of higher pressure to another of lower magnitude, when the pressure difference or gradient is maintained throughout the cardiac cycle.
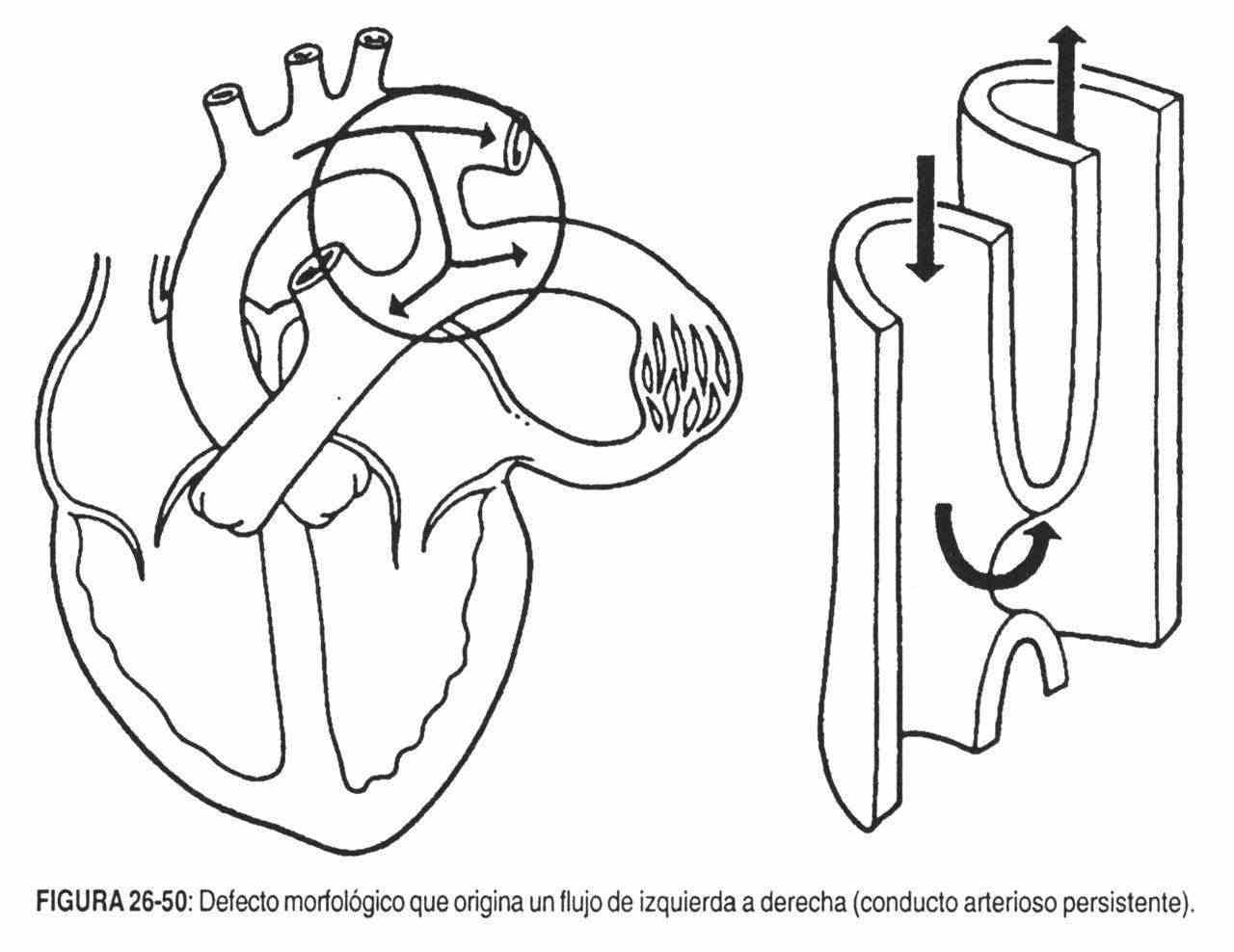 In the most common cases (patent ductus arteriosus), the current is retrograde from the left to the right circulation through a morphologic defect (Figure 26-50). Therefore, they are regurgitation murmurs, with a systolic and a diastolic element.
In the most common cases (patent ductus arteriosus), the current is retrograde from the left to the right circulation through a morphologic defect (Figure 26-50). Therefore, they are regurgitation murmurs, with a systolic and a diastolic element.
Precisely by virtue of the fusion of both elements of regurgitation, continuous murmurs have the common characteristic of reaching their maximum intensity around the second sound.
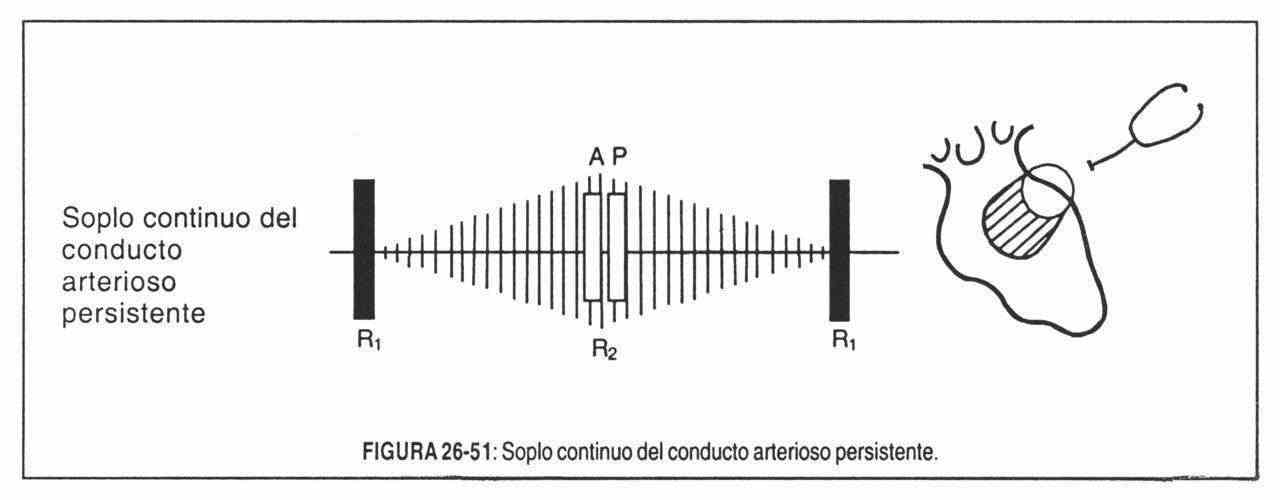 The most common condition that can lead to a continuous thoracic murmur is the patent ductus arteriosus (Figure 26-51), also called the Gibson murmur or machine murmur. It is of high frequency, strong, generally with thrill, maximum in the first or second left intercostal space, parasternal. If it is more intense in the left third intercostal space than in the left first intercostal space, the diagnosis should be doubted. The most intense systolic component radiates to the precordium, to the left supraclavicular fossa, and sometimes to the dorsum, in the left interscapulovertebral space
The most common condition that can lead to a continuous thoracic murmur is the patent ductus arteriosus (Figure 26-51), also called the Gibson murmur or machine murmur. It is of high frequency, strong, generally with thrill, maximum in the first or second left intercostal space, parasternal. If it is more intense in the left third intercostal space than in the left first intercostal space, the diagnosis should be doubted. The most intense systolic component radiates to the precordium, to the left supraclavicular fossa, and sometimes to the dorsum, in the left interscapulovertebral space
It is a murmur that is accentuated in late systole and in early diastole (around the second sound) and fades in the second half of diastole.
Although patent ductus arteriosus is the most common cause of continuous precordial murmur, it should not be forgotten that "not all patent ductus arteriosus produce a continuous murmur, nor are all continuous murmurs due to the persistence of the ductus." Thus, we will mention - by way of example - other causes: the aortopulmonary window, aneurysm of the sinus of Valsalva ruptured in the right cavities, coronary fistulas, creation of a surgical artificial ductus (Blalock's operation in the tetralogy of Fallot), arteriovenous fistulas pulmonary and systemic, breast murmur of pregnancy, etc.
Innocent murmurs. Those murmurs that are not accompanied by underlying causal cardiac pathology are thus named. These audible phenomena occur in practically everyone at some point in their lives, and their recognition is very important, as much or more than the recognition of a significant murmur of disease.
There is a series of data common to all innocent murmurs: they are very frequent in children, not very intense, without thrill, sometimes musical, brief, never pansystolic and never diastolic.
Innocent lung murmurs . They are an exaggeration of the normal expulsive vibrations that originate in the pulmonary artery. They are very frequent in children, to the point that some authors find them in almost 100% of cases. In other words, in children an innocent lung murmur is the rule and not the exception.
These are weak murmurs, with a normal second sound, early, short systolic rhomboid and that diminish or disappear on deep inspiration. Sometimes they are maximum in the decubitus, and others with the patient upright. They can radiate to the mitral area and the aortic area.
Innocent aortic murmurs . They are the most common murmurs in adults above middle age, and are best referred to as "aortic valve sclerosis" murmurs. They represent the benign end of a spectrum, the pathological end of which is calcific aortic stenosis. Its cause is the slight fibrous -or fibrocalcic- thickening of the bases of the aortic leaflets in its aortic face, at the level of its insertion in the sinuses of Valsalva. Otherwise, the leaves are normal, and their free edges move normally. They are mid-systolic ejection murmurs, usually faint, exceptionally initiated by an ejection click or sound.
Vibratory or Still's murmur . It's very typical. It is heard in the mesocardium or apex in about 50% of children. Its low-pitched musical vibratory timbre is very distinctive, reminiscent of the buzz that occurs when a fork vibrates.
It begins immediately after the first noise, is rhomboid, with an early vertex, and ends at mesosystole. It probably originates from the periodic vibrations of the pulmonary blades at their insertions.
Parasternal murmur . High-frequency systolic murmur seen in certain thoracic deformities such as the pectum excavatum. It is protomesosystolic, low parastemal, without thrill. It does not disappear with change of position or with respiratory movements. Its genesis probably involved displacements of the heart and traction of the great vessels.
Apical protosystolic murmur . It is a soft, 1/6 to 2/6, decreasing, proto-systolic murmur, heard at the apex or endoapex and not radiating. It is probably an expulsive intracardiac murmur, produced by accelerated flow in the right or left ventricle. It is found in children and young people.
"Hemic" murmurs . They are due to an abnormal increase in flow velocity. They appear in the so-called "hyperkinetic states" of the circulation (anemia, hyperthyroidism, etc.), with high cardiac output. These murmurs have the same characteristics as the innocent lung murmur in children and, occasionally, the apical proto-systolic murmur.
Carotid or brachiocephalic murmurs . It can be heard in children, on the right side of the neck. It is late systolic and occurs at the site of the brachiocephalic trunk bifurcation in the carotid and subclavian artery. Disappears in cervical hyperextension.
Venous buzzing . It is a continuous anorganic murmur, produced by the rapid passage of blood from the jugulars to the innominate trunks. It is heard mainly in children, and is located in the upper and anterior portion of the thorax and at the base of the neck. It has diastolic reinforcement and is usually of medium intensity.
It is very common in anemia.
It is heard almost exclusively standing up, varies in successive cardiac cycles, and disappears when the head is rotated or when the base of the neck is compressed with the finger. These are its typical characteristics.
Murmur breast . It is a continuous murmur located in the second or third intercostal space on both sides of the sternum. It is due to the turbulence caused in the internal and intercostal mammary arteries due to the hyperflow existing in the last three months of pregnancy and in the first months after delivery. It is heard in a sitting position, and can disappear standing up.